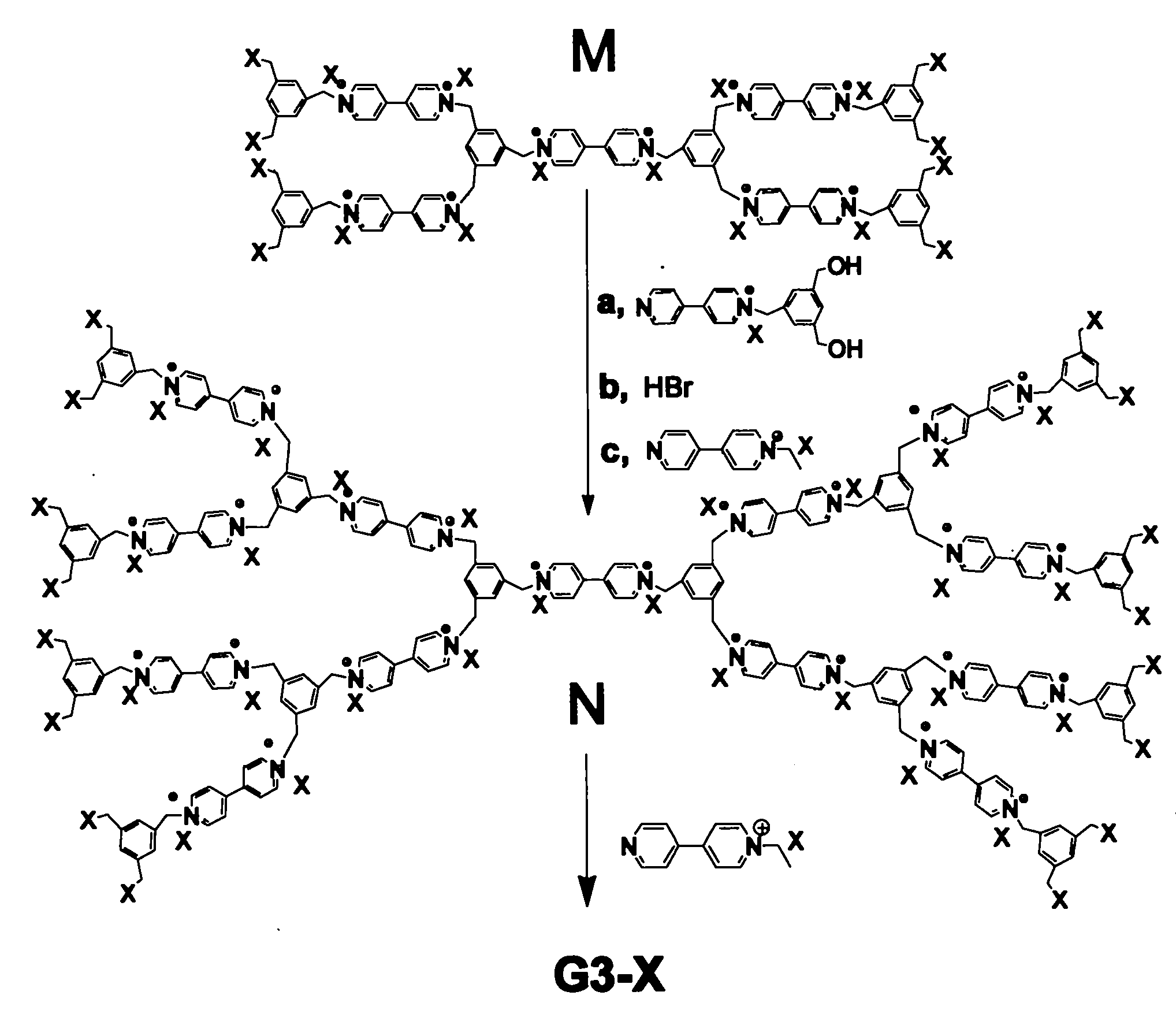Solar cell gel electrolyte and preparation method and application thereof
A gel electrolyte and solar cell technology, applied in the field of solar cells, can solve problems such as low cell efficiency, and achieve the effects of high cell efficiency, favorable migration, and resistance to leakage and combustion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
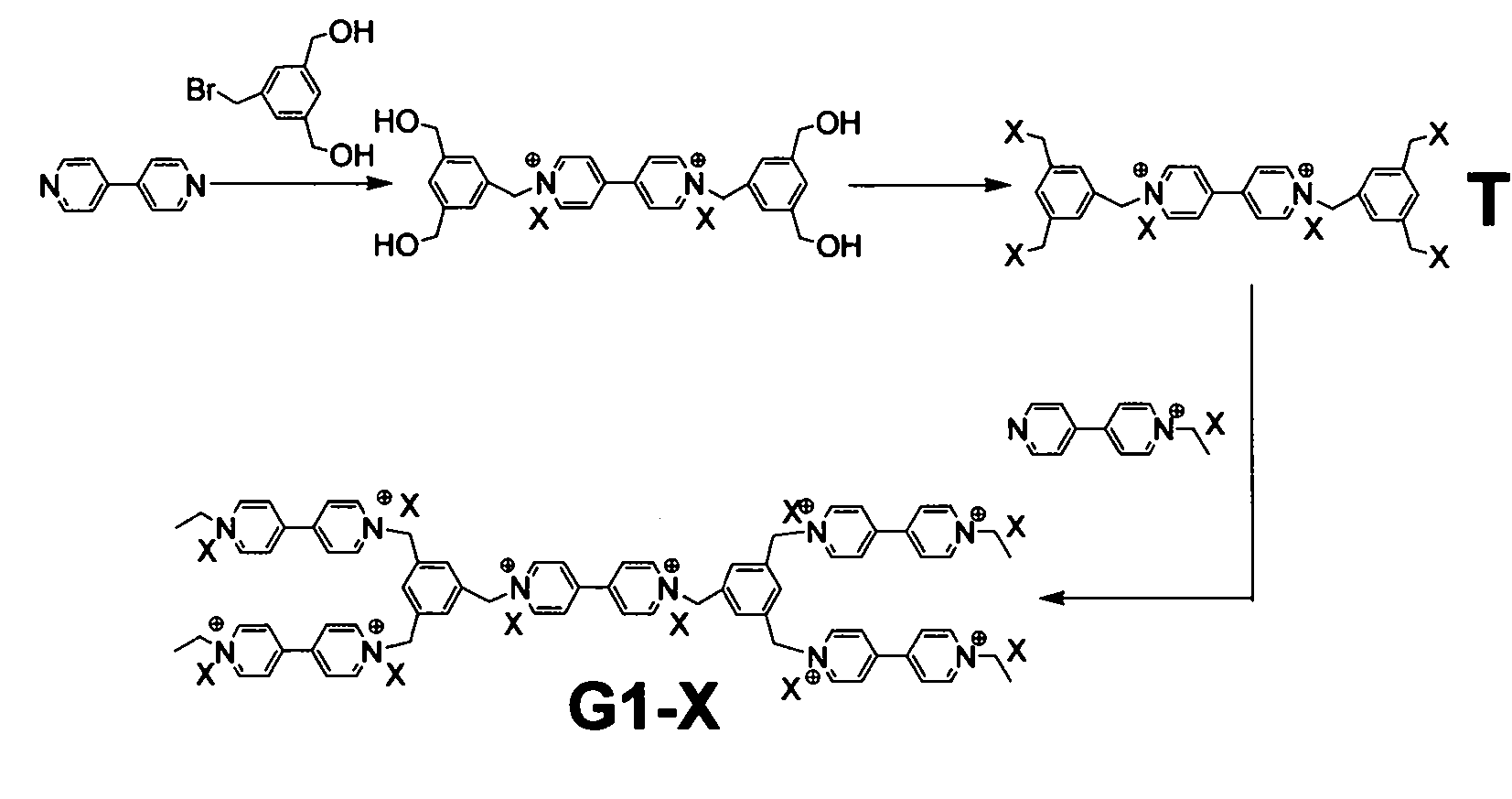
[0047] Preparation of G1-Br: see figure 1 , take 2.5 servings Add 1 part Stir at room temperature for two days, wash with ether three times to remove unreacted raw materials, and then remove the solvent. Add appropriate amount of hydrogen bromide and acetic acid, and react at 60°C for 6h, then remove the raw materials and solvent. Then dissolve with ethanol, add 2 parts After fully reacting at 60°C for 2 days, the ethanol was removed and dried in vacuum. That is the product G1-Br.
Embodiment 2
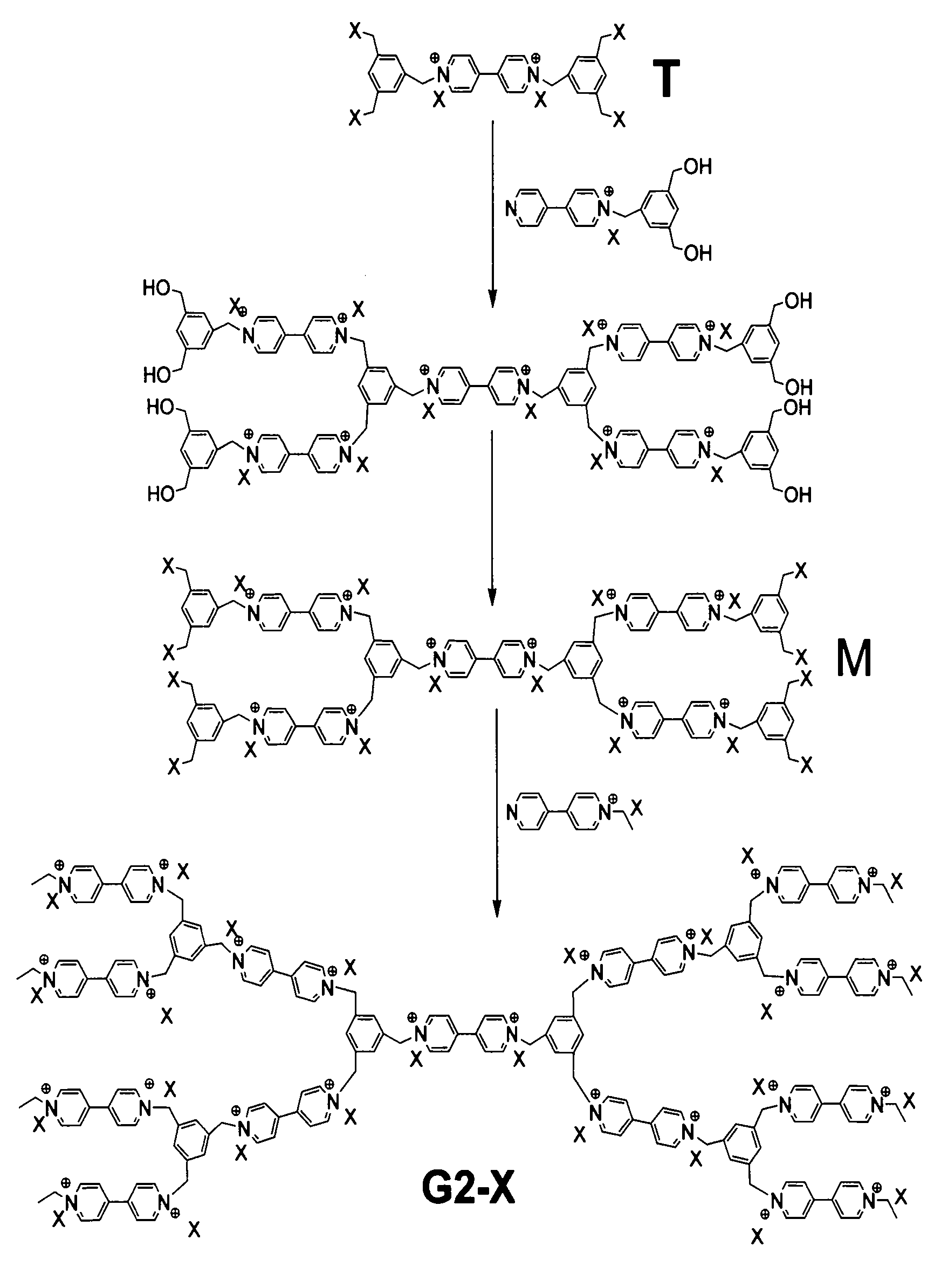
[0049] Preparation of G2-Br: see figure 2 , take 1 copy Added 4.5 servings Ethanol was used as the solvent, and stirred at 60°C for two days. After removing the ethanol, it was washed with ether three times to remove unreacted raw materials, and then the solvent was removed. Add an appropriate amount of hydrogen bromide and acetic acid, and react at 60°C for 8 hours, then remove the raw materials and solvent. Then dissolve with ethanol, add 2 parts After fully reacting at 60°C for 2 days, the ethanol was removed and dried in vacuum. That is the product G2-Br.
Embodiment 3
[0051] Preparation of G2-TFSI: see image 3 , Take 1 part of G1-Br, dissolve it in deionized water, add 1.2 parts of lithium bistrifluoromethanesulfonimide (LiTFSI), stir at room temperature for 12 hours, and remove the water. The product G2-TFSI was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com