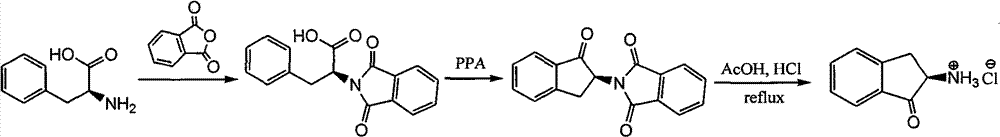
Synthesizing method of chiral amino indanone
A synthesis method and amino technology, applied in chemical instruments and methods, preparation of organic compounds, production of bulk chemicals, etc., can solve the problems of long route and harsh reaction conditions, and achieve the effect of short synthesis route and excellent synthesis method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Weigh 3g of N-phthaloyl-L-phenylalanine into a 250mL round-bottomed flask with a magnetic stirrer, add 20ml of polyphosphoric acid, put it in an oil bath, install a reflux device, and put it in an oil bath Heat up to 130°C, react for 3 hours; then cool to room temperature, add 60ml of water to the flask, add 30ml of CH 2 Cl 2 Extracted 3 times, the organic phase was extracted with anhydrous MgSO 4 Dry and filter, and the filtrate was removed from the solvent on a rotary evaporator to obtain a light yellow solid; column chromatography was performed with an eluent of petroleum ether / ethyl acetate (volume ratio 4:1) to obtain a white solid (S)-2- Phthalimido-1-indanone 2.1030g, the yield is 74.6%; [α] D 23 =-21.5 (c 0.46, chloroform); melting point is 203-205°C; IR, v (cm -1 ): 1723, 1602, 1461, 1446, 1383, 1266, 1115, 1074, 1048, 974, 872, 760, 706, 643; 1 H NMR (300MHz, CDCl 3 ), δ (ppm): 3.40 (dd, J 1 =16Hz,J 2 =6Hz, 1H), 3.60(dd, J 1 =16Hz,J 2 =8.4Hz, 1H), 5....
Embodiment 2
[0013] Similar to the operation of Example 1, N-phthaloyl-D-phenylalanine was used as the raw material to obtain 2.2101 g of (R)-2-phthalimide-1-indanone as a white solid, producing The rate is 78.4%; [α] D 23 =-20.8 (c 0.6, chloroform); melting point is 202-204°C; IR, 1 H NMR agrees.
Embodiment 3
[0015] Similar to the operation of Example 1, N-phthaloyl-DL-phenylalanine was used as the raw material to obtain 2.0800 g of (±)-2-phthalimido-1-indanone as a white solid, producing The rate is 73.8%; the melting point is 193-195°C; IR, 1 H NMR agrees.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com