Method for screening cathepsin B inhibitor by adopting ultra performance liquid chromatography and mass spectrometry
A cathepsin, ultra-high performance liquid phase technology, applied in biochemical equipment and methods, microbial determination/inspection, measurement devices, etc. High accuracy, rapid sample detection, and the effect of avoiding false positive and false negative results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The method for screening cathepsin B inhibitors by ultra-high performance liquid chromatography and mass spectrometry provided by the invention comprises the following steps:
[0054] (1) Preparation of enzymatic reaction buffer
[0055] The buffer solution includes 0.4 mol / L sodium acetate, 4 mmol / L EDTA, 8 mmol / L dithiothreitol, and the pH value is adjusted to 5.5 with acetic acid;
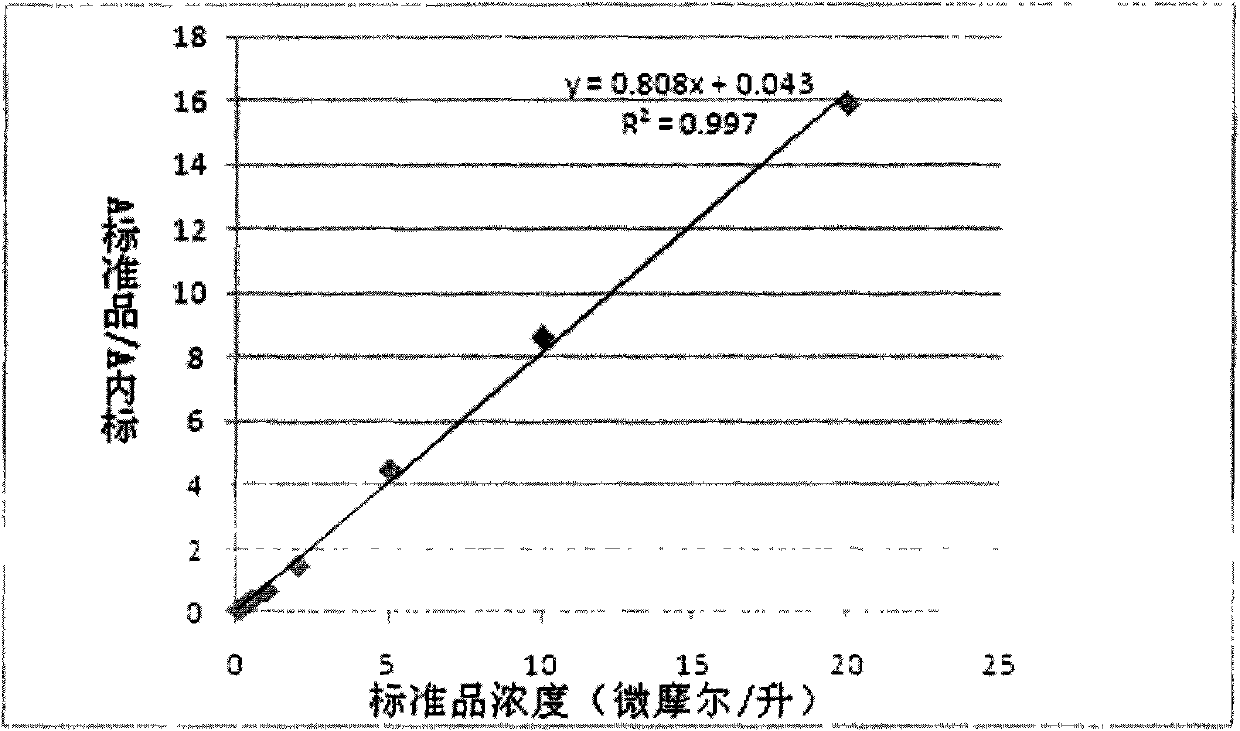
[0056] Preparation of 7-amino-4-methylcoumarin standard substance: use methanol to make 7-amino-4-methylcoumarin standard substance into concentrations of 100 nanomoles / liter, 200 nanomoles / liter, 500 nanomoles / liter respectively Nanomol / L, 1μmol / L, 2μmol / L, 5μmol / L, 10μmol / L, 20μmol / L standard solutions, and each concentration of the standard contains 1μmol / liter of 7-amino-4-methoxymethyl coumarin as internal standard;
[0057] (2) Preparation of standard products
[0058] Use methanol to prepare the standard products with concentrations of 100 nanomoles / liter, 200 nanomoles / liter,...
Embodiment 2
[0090] The sample is as follows: the total reaction volume is 100 microliters, the final concentration of cathepsin B is 8 nanomoles / liter, and the standard product of ligustrazine with a final concentration of 100 micromoles / liter is incubated at 37 degrees Celsius for 30 minutes, and a final concentration of 20 micromoles / liter is added. Substrate, react at 37 degrees Celsius for 20 minutes, add 1% trifluoroacetic acid to terminate the reaction, and add 1 micromol / liter internal standard at the same time, as a sample for ultra-high performance liquid chromatography / mass spectrometry;
[0091] All the other are with embodiment 1;
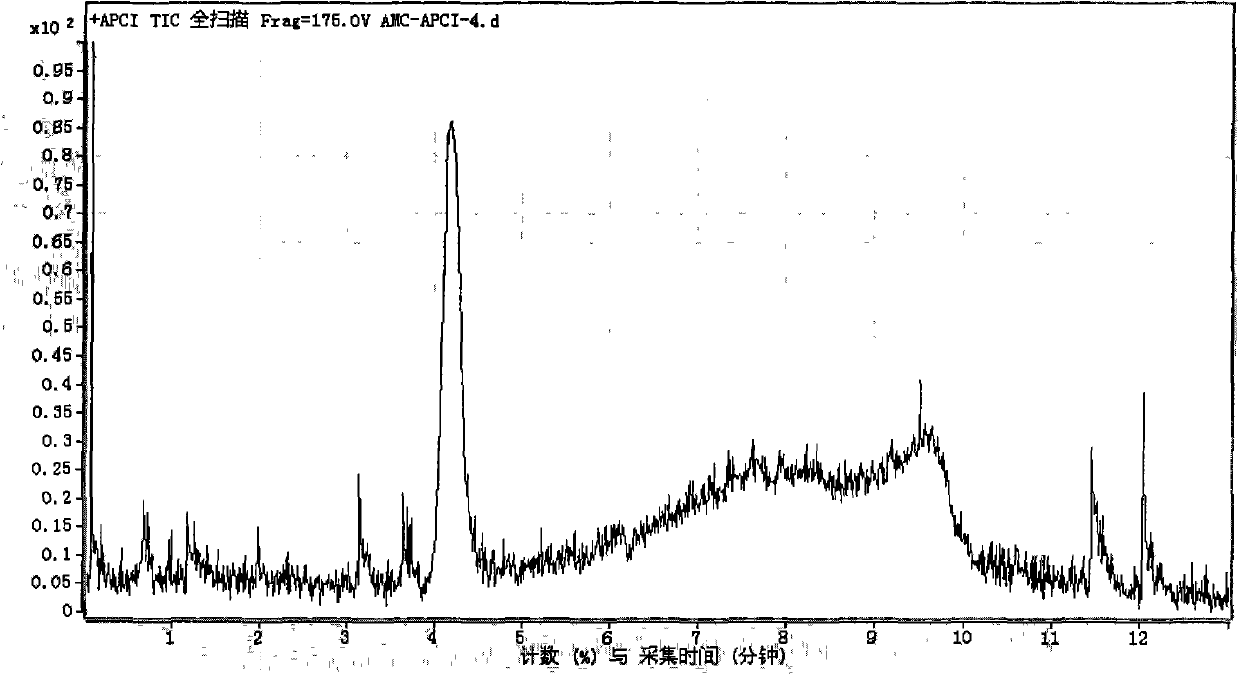
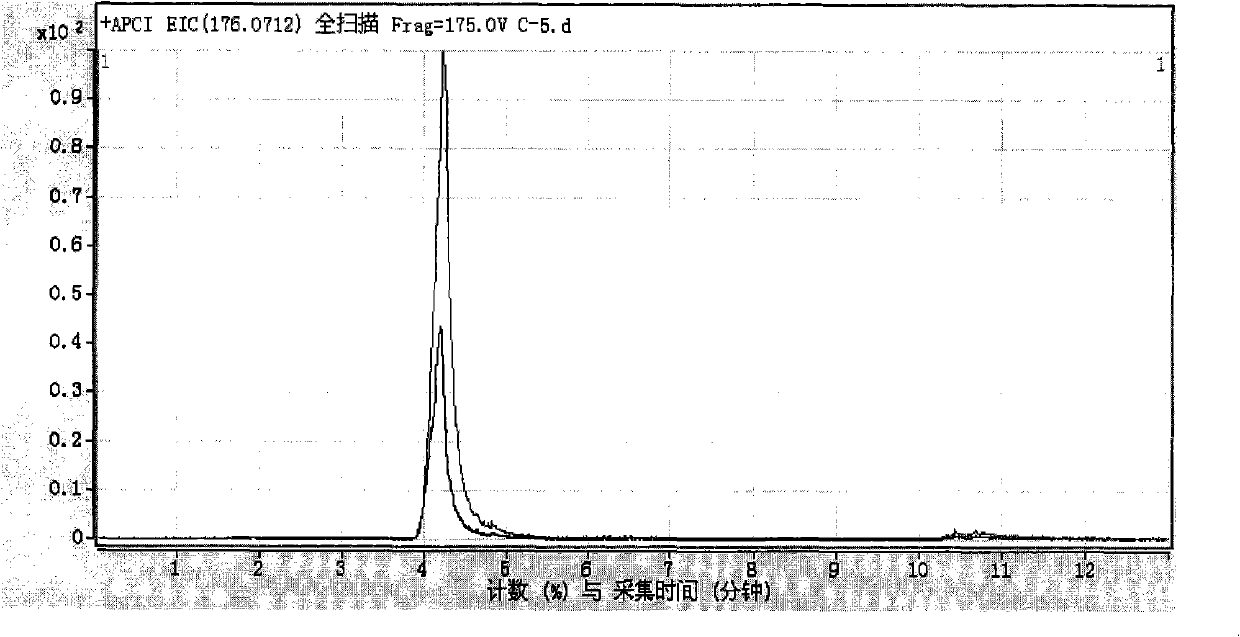
[0092] According to the detection steps of Example 1, the inhibition rate of cathepsin by 1 μmol / L ligustrazine standard substance was detected and calculated to be 9%. Figure 6 It is the extraction chromatogram of sample in embodiment 2.
Embodiment 3
[0094] The sample is as follows: the total reaction volume is 100 microliters, the final concentration of cathepsin B is 8 nanomoles / liter, and the final concentration of 100 micromoles / liter tanshinone IIA standard is incubated at 37 degrees Celsius for 30 minutes, and the final concentration of 20 micromoles / liter is added. Substrate, react at 37 degrees Celsius for 20 minutes, add 1% trifluoroacetic acid to terminate the reaction, and add 1 micromol / liter internal standard at the same time, as a sample for ultra-high performance liquid chromatography / mass spectrometry;
[0095] All the other are with embodiment 1;
[0096] According to the detection procedure of Example 1, the inhibition rate of cathepsin by 1 micromole / liter tanshinone IIA standard substance was detected and calculated to be 1%. Figure 7 It is the extraction chromatogram of sample in embodiment 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com