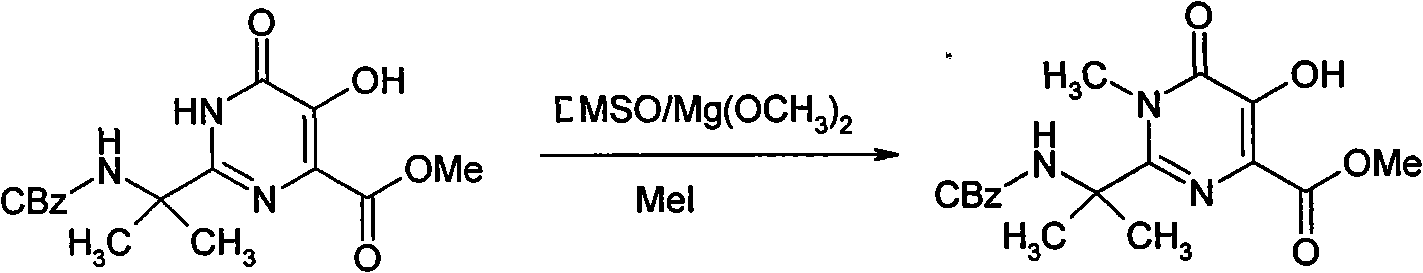Method for synthesizing N-methyl pyrimidone
A technology of methylpyrimidinone and synthesis method, applied in the field of chemical synthesis, to achieve the effect of simplifying post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
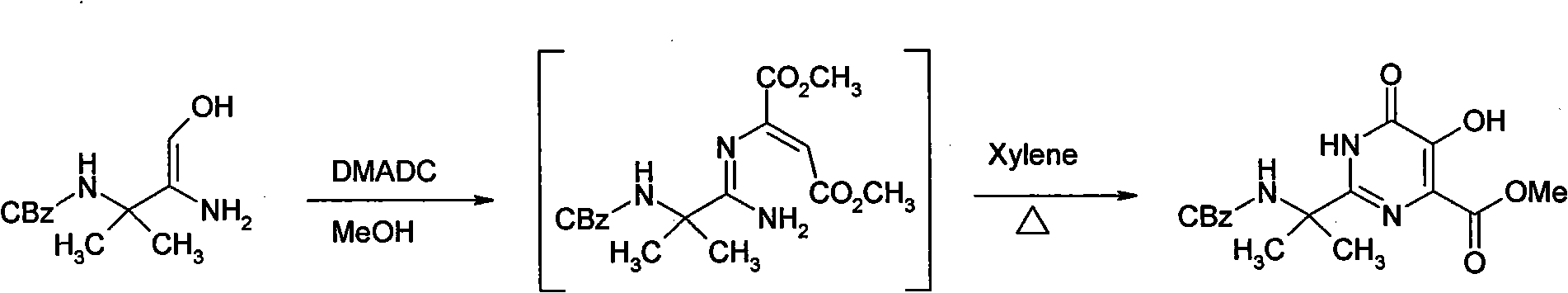
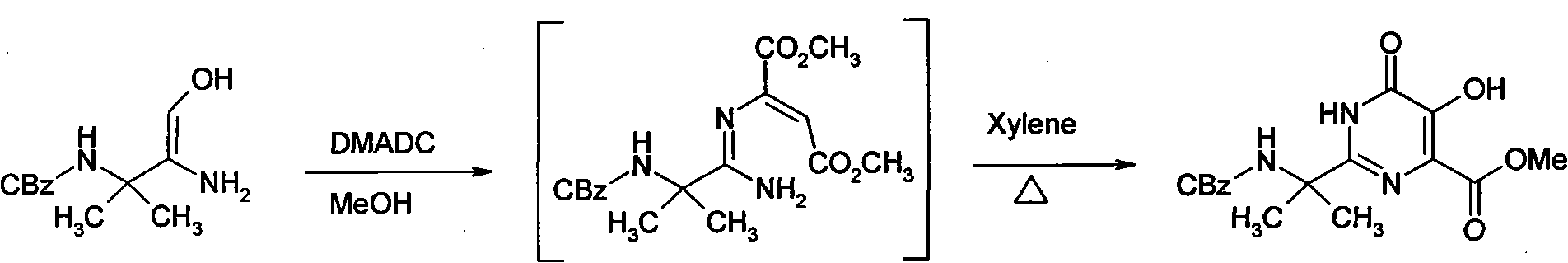
[0015] Embodiment 1 synthetic hydroxypyrimidinone
[0016]
[0017] Suspend 5.8 g of amidoxime in 24 ml of methanol, and add 3.13 g of dimethyl acetylenedicarboxylate dropwise. Stir at 30° C. for 1.5 hours. After the reaction was detected by TLC, about 16 ml of methanol was evaporated under reduced pressure, then 30 ml of xylene was added, and about 15 ml was evaporated under reduced pressure. Finally, the temperature was raised to reflux (above 125° C.) for 5 hours. Allow to cool below 60°C, add 3ml of methanol, then gradually add 30ml of methyl tert-butyl ether, stir evenly, cool to 0-5°C, and keep warm for 5 hours. The precipitated crystals were filtered, and then the mother liquor was cooled to 0° C. and kept for 5 hours. Filter the crystallization that separates out, merge with next time product, obtain 5.8g hydroxypyrimidinone product altogether, productive rate 69.5%.
Embodiment 2
[0018] The N-methylation of embodiment 2 hydroxypyrimidinones
[0019]
[0020] 23g of hydroxypyrimidinone was dissolved in 260ml of pyridine, and 29.32g of benzoic anhydride was added dropwise. Stir overnight at room temperature. The solvent was distilled off under reduced pressure, and the residue was dissolved in 30 ml of ethyl acetate, washed successively with dilute hydrochloric acid, sodium bicarbonate solution and brine, and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain 21 g of a brown product.
[0021] To a solution of 0.34 g of lithium hydride in 178.5 ml of dioxane was added the benzoyl-protected hydroxypyrimidinone obtained previously. The mixture was aged at 38°C for 1 hour and cooled to room temperature. Add 5ml of dimethyl sulfate and heat at 55-60°C for 4 hours. Cool to room temperature, add 1 drop of glacial acetic acid, then add 200ml of water and 200ml of ethyl acetate. After shaking, the two phase...
Embodiment 3
[0022] The N-methylation of embodiment 3 hydroxypyrimidinones
[0023]
[0024] Dissolve 14.0 g of benzoyl-protected hydroxypyrimidinone in 700 ml of dry tetrahydrofuran, add 5.2 g of cesium carbonate and 4.04 ml of dimethyl sulfate, and stir at 60° C. for 1 hour. The solvent was evaporated from the reaction mixture, and 200 ml of ethyl acetate was added to dissolve it. Washed successively with dilute hydrochloric acid, water and brine, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure, the crude N-methylated product obtained was treated in the same manner as in Example 2 to obtain 4.6 g of N-methylpyrimidinone, Yield 41%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com