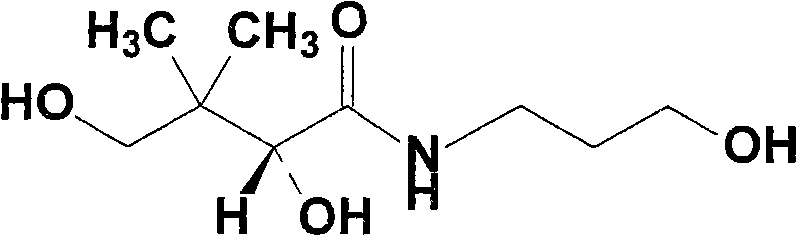
Dexpanthenol infusion preparation and preparation method thereof
A technology of dexpanthenol and its preparations, which is applied in the field of dexpanthenol infusion preparations and its preparation, can solve the problems of dexpanthenol infusion preparation research and market reports that have not been seen, and achieve simple and feasible operation, controllable quality, and overcome the pH value unstable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
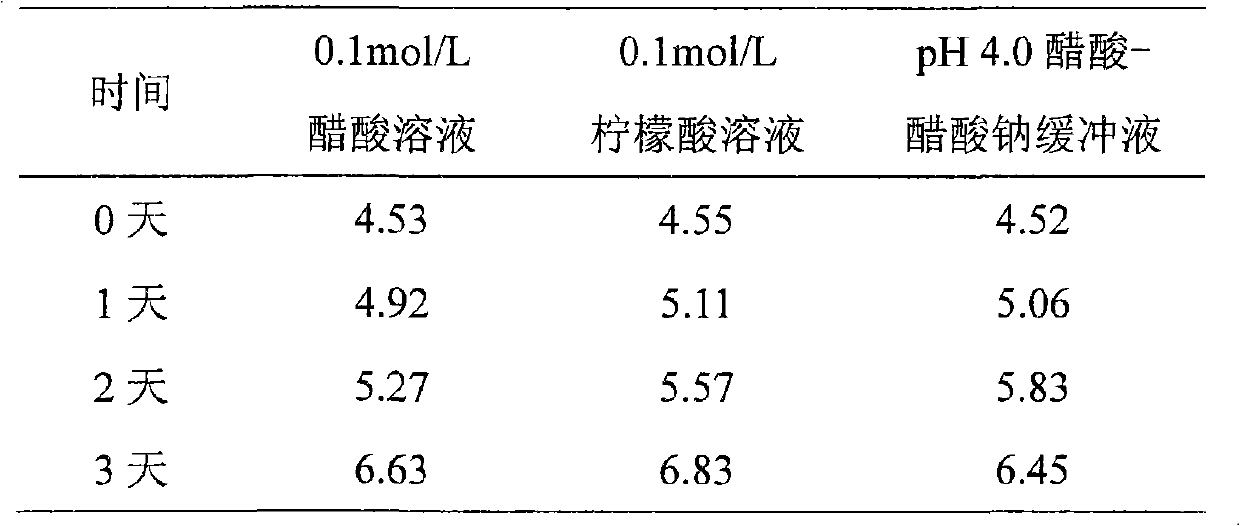
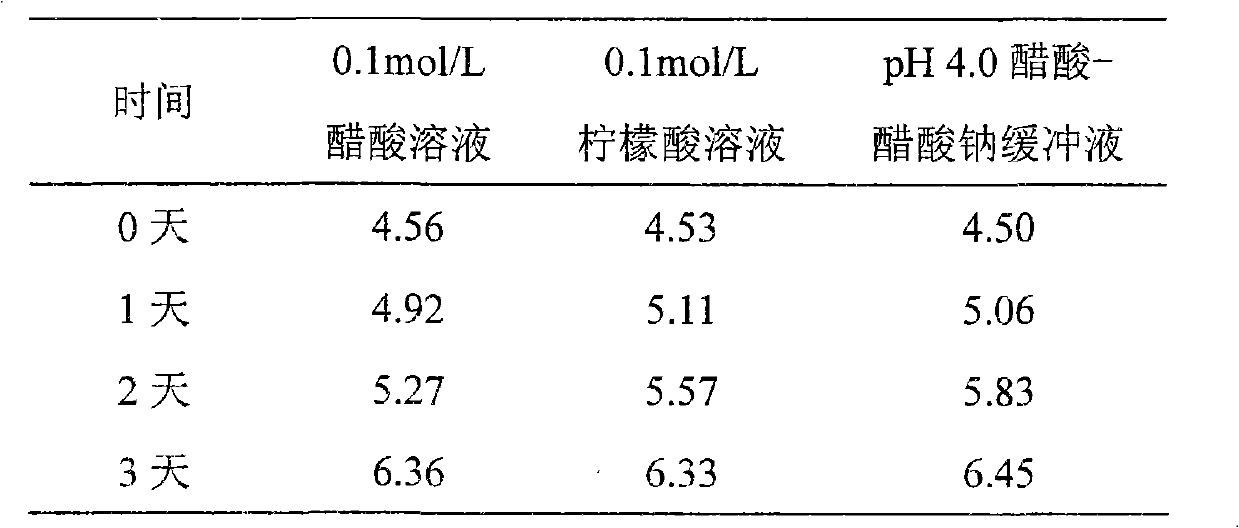
[0076] Weigh 11.8g of sodium acetate trihydrate, add an appropriate amount of water for injection to dissolve; measure 2.8ml of glacial acetic acid and add, quantify to 2,000ml, and measure the pH value to be 4.01. Weigh 15g of sodium chloride and add, stir until completely dissolved.
[0077] Weigh 10g of dexpanthenol, add the above solution to about 1,600ml, stir until completely dissolved; take 0.01% activated carbon for needles, stir at room temperature for 15min and then filter; Sodium solution, so that the concentration is 1ml: 5mg; sterilized by filtration through a 0.22μm microporous membrane; potting, the volume of each bottle is 100ml; sterilized by autoclaving at 121°C for 8 minutes, inspected, printed, and packaged.
Embodiment 2
[0079] Weigh 2.14g of sodium acetate trihydrate, add an appropriate amount of water for injection to dissolve; measure 1.6ml of glacial acetic acid, add it to 2,000ml, and measure the pH value to 4.51. Weigh 15g of sodium chloride and add, stir until completely dissolved.
[0080] Weigh 10g of dexpanthenol, add the above solution to about 1,600ml, stir until completely dissolved; take 0.05% activated carbon for needles, stir at room temperature for 20min and then filter; Sodium solution, so that the concentration is 1ml: 5mg; sterilized by filtration through a 0.22μm microporous membrane; potting, the volume of each bottle is 100ml; sterilized by autoclaving at 121°C for 12min, inspected, printed, and packaged.
Embodiment 3
[0082] Weigh 3.38g of sodium acetate trihydrate, add an appropriate amount of water for injection to dissolve; measure 0.8ml of glacial acetic acid, add it to 2,000ml, and measure the pH value to 4.98. Weigh 15.2 g of sodium chloride and add, and stir until completely dissolved.
[0083] Weigh 10g of dexpanthenol, add the above solution to about 1,600ml, stir until completely dissolved; take 0.10% activated carbon for needles, stir at room temperature for 25min and then filter; Sodium solution, so that the concentration is 1ml: 5mg; sterilized by filtration through a 0.22μm microporous membrane; potting, the volume of each bottle is 100ml; sterilized by autoclaving at 121°C for 15 minutes, inspected, printed, and packaged.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com