Device and method for continuously analyzing single-cell contents by miniflow control chip at high speed
A microfluidic chip and content technology, applied in the field of single-cell analysis, can solve problems such as not being widely used, and achieve the effects of increasing the injection rate, reducing the concentration, and having a simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
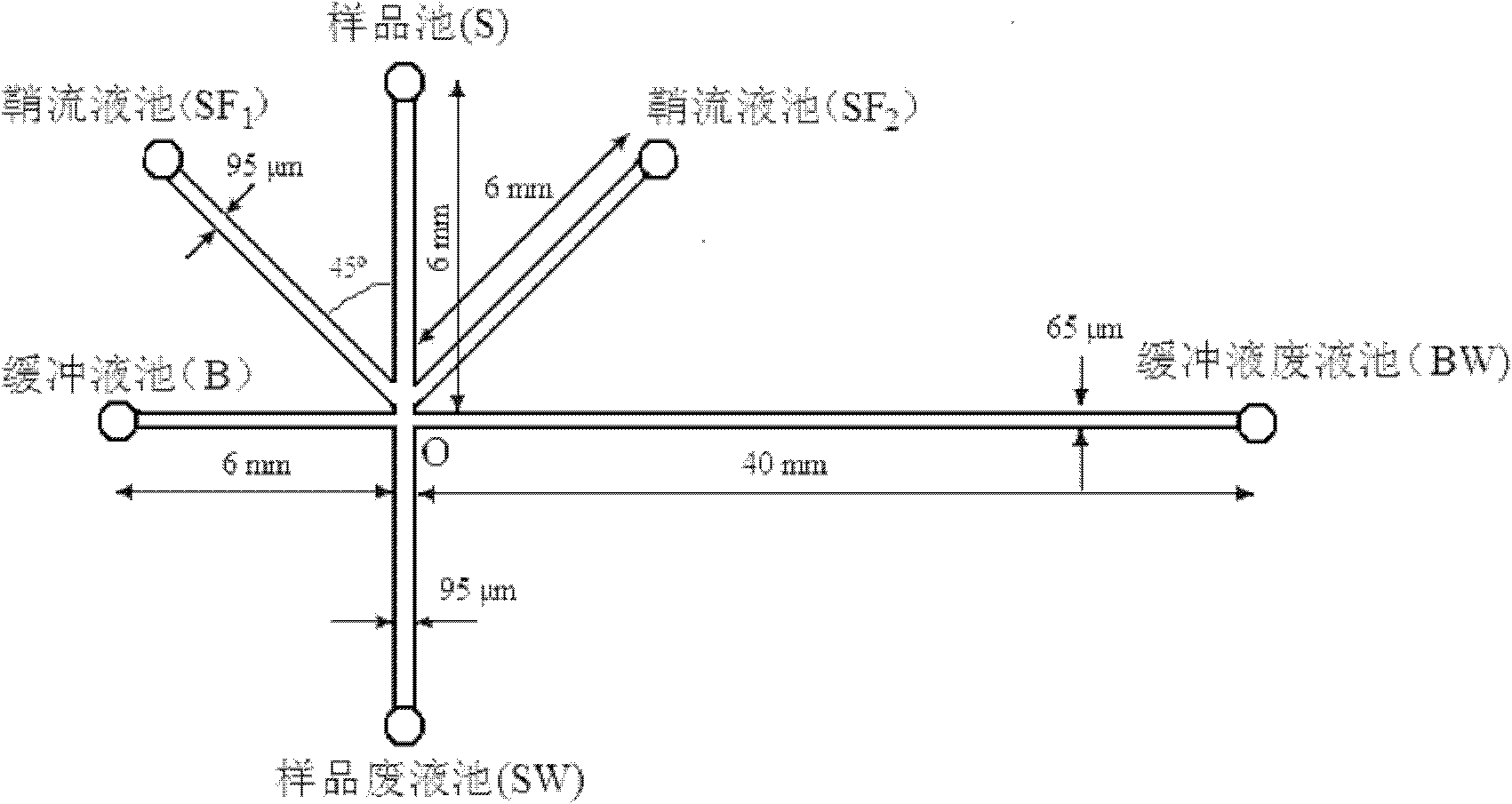
[0022] see figure 1 , the microfluidic chip has a buffer pool (B), a sample pool (S), a sample waste pool (SW), two sheath flow pools (SF 1 and SF 2 ) and buffer waste reservoir (BW). The sampling channel is S-SW, the length is 12 mm, the channel width is 95 μm, the depth is 35 μm, the length of channel S-O is 6 mm; the separation channel is B-BW, the length is 46 mm, the channel width is 65 μm, the depth is 20 μm, and the length of channel B-O is 6 mm; The sample channel crosses the separation channel at point O. There is a sheath flow channel on both sides of the sampling channel, which intersects with the sampling channel at 200nm above point O. The sheath flow channel is 95 μm wide, 35 μm deep, and 6 mm long. A small hole is punched at the end points of the sampling channel, the separation channel and the sheath flow channel, and the micro plastic liquid reservoir is glued on the small hole with an adhesive.
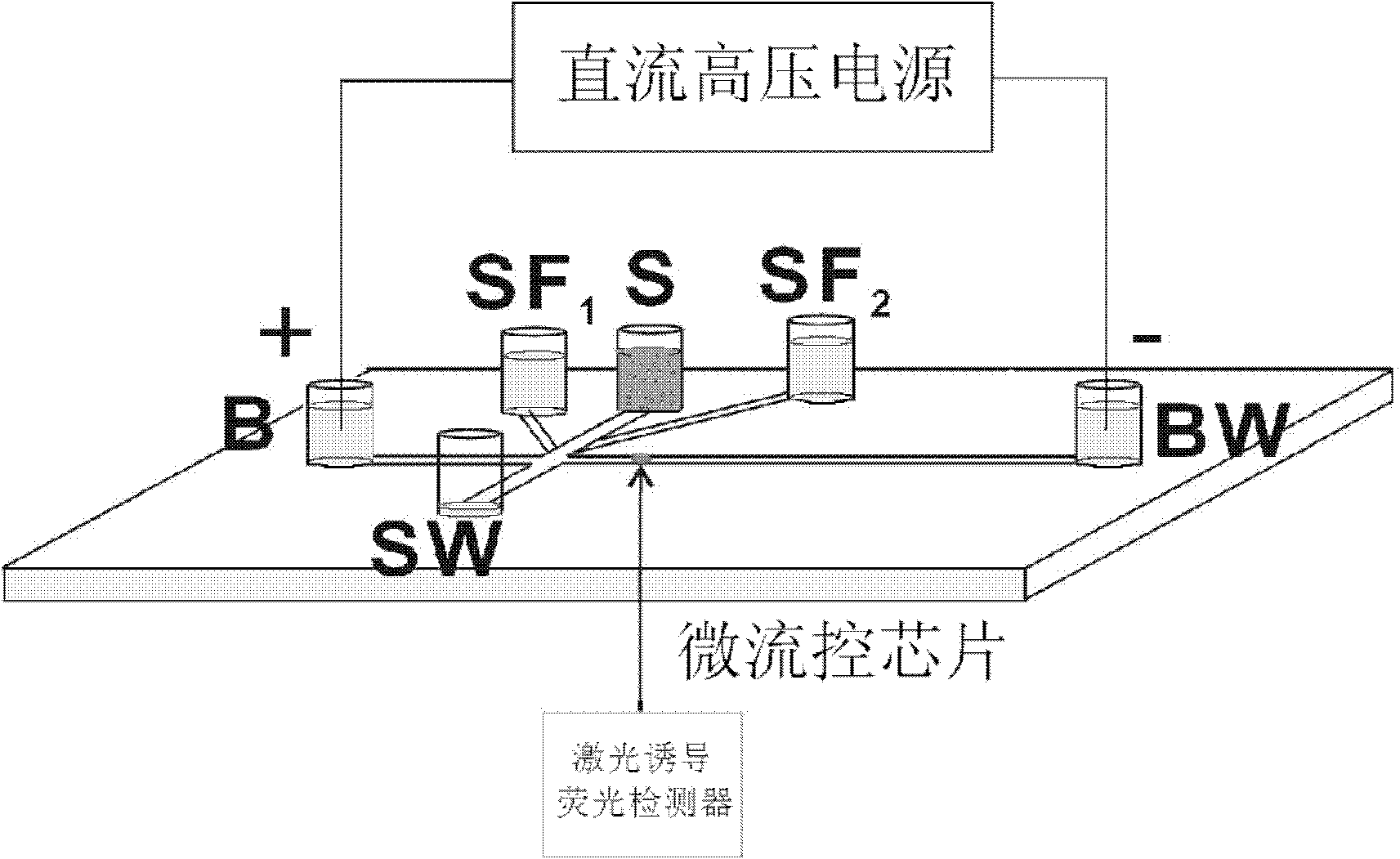
[0023] see figure 2 Add 100 μL and 100 μL of electrophore...
Embodiment 2
[0025] Example 2: Determination of glutathione and reactive oxygen species in a single red blood cell
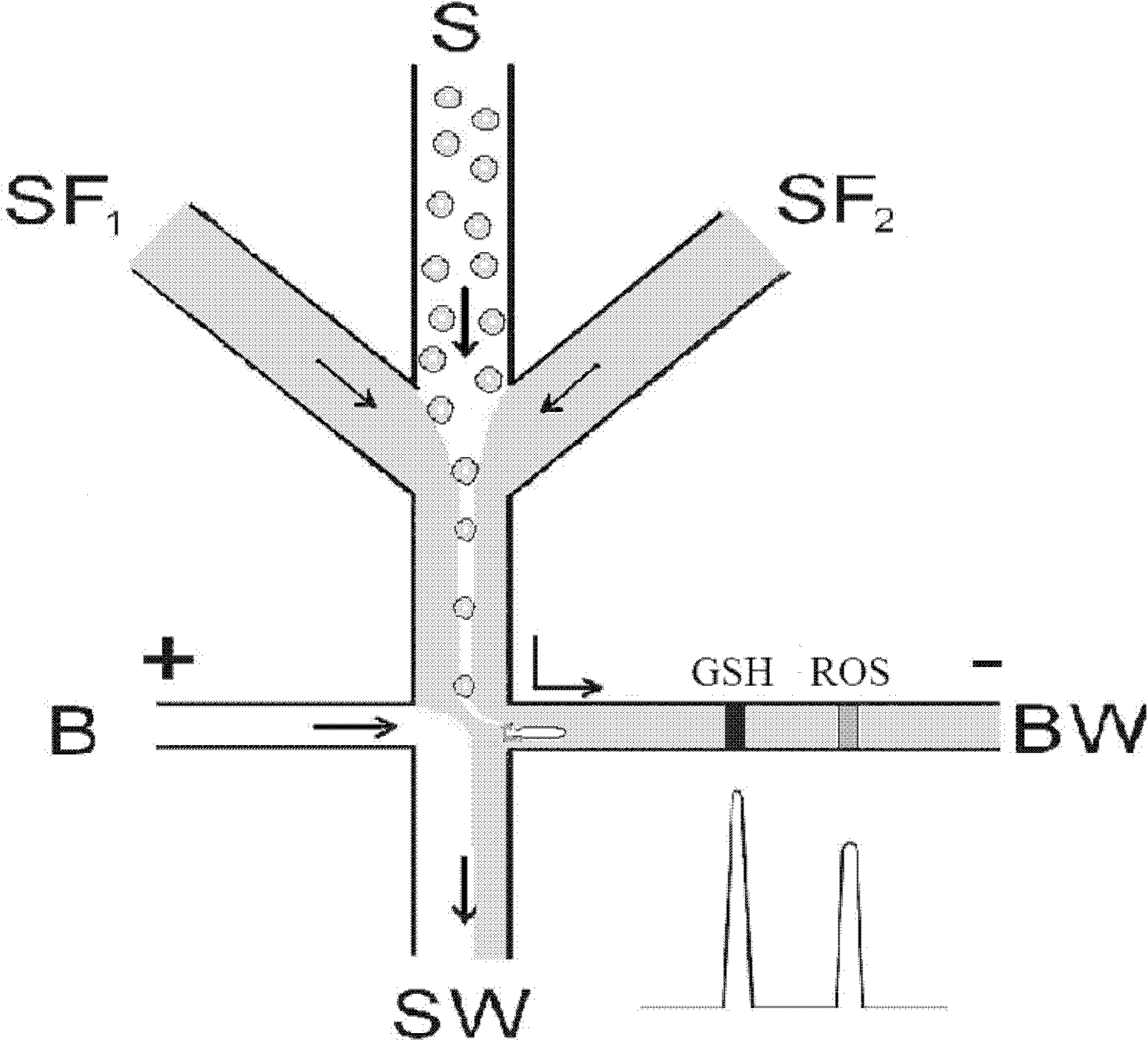
[0026] The microfluidic chip used in this example is consistent with Example 1, and the sample is red blood cell suspension. Label glutathione and reactive oxygen species in cells with naphthalene dicarbaldehyde and dihydrorhodamine 123, respectively, and dilute with normal saline to prepare a density of 1.2×10 5 After the cells / mL cell suspension was added to the sample pool; 20mmol boric acid buffer solution (pH 9.2) was added to the reservoirs B and BW, and the electrophoresis buffer containing the film dissolving agent (tritonX-100, concentration 1%, w / w) solution (20mmol boric acid buffer solution, pH 9.2) into the sheath flow reservoir SF 1 and SF 2 In, the amount of liquid added in each reservoir is consistent with that of Example 1. Apply a 2000V DC voltage to the B and BW reservoirs at the two ends of the separation channel.
[0027] Cells in the erythrocyte sus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com