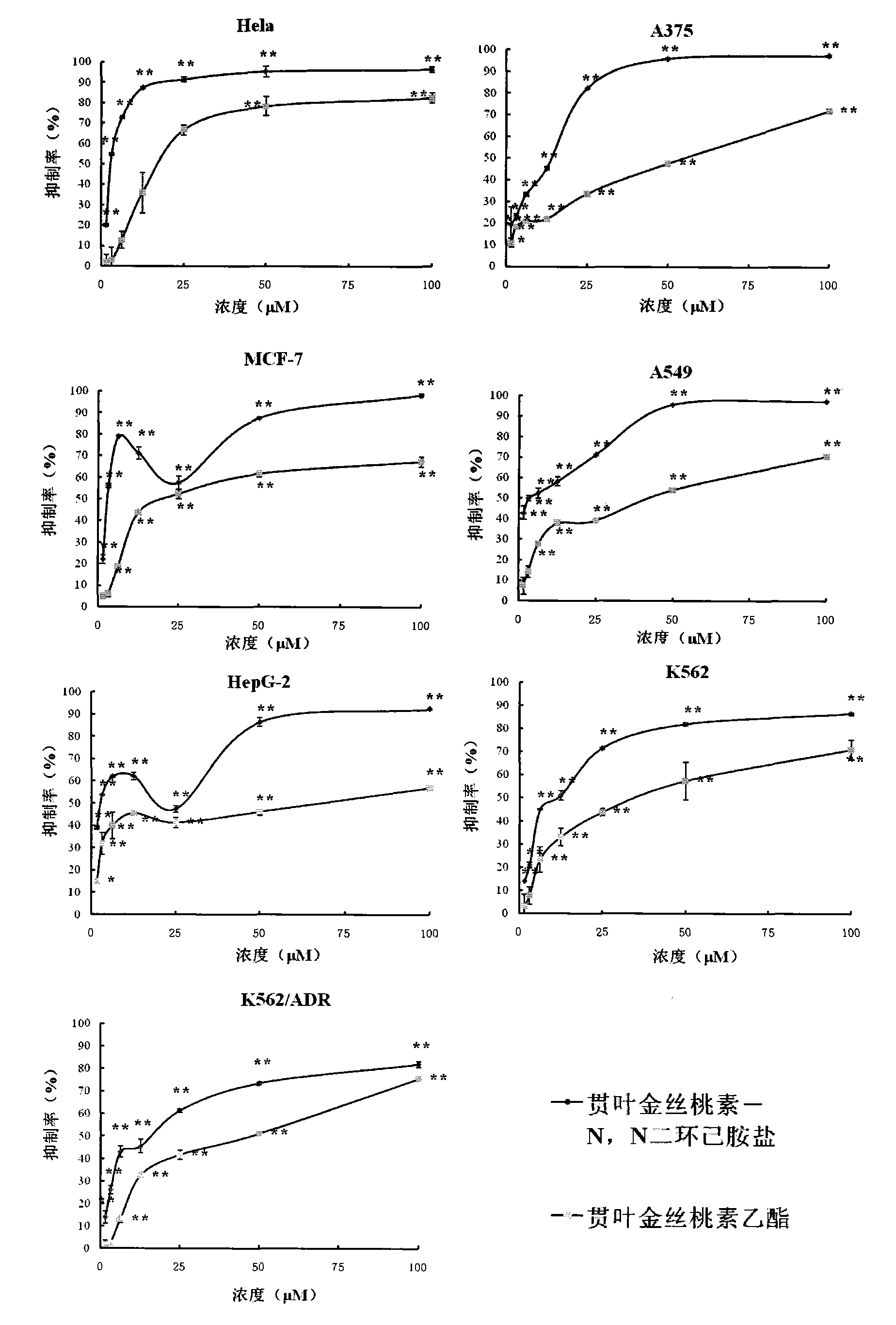
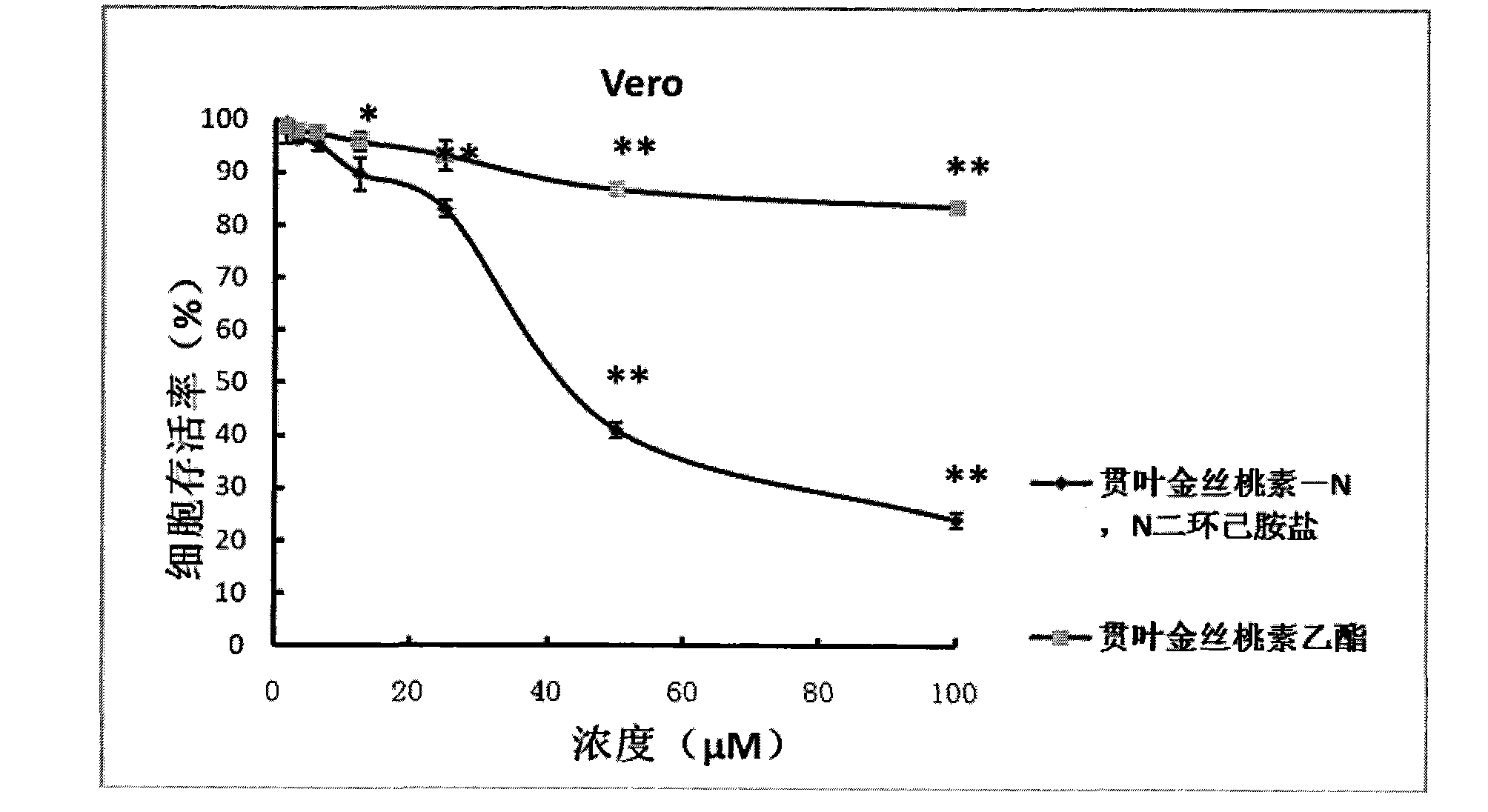
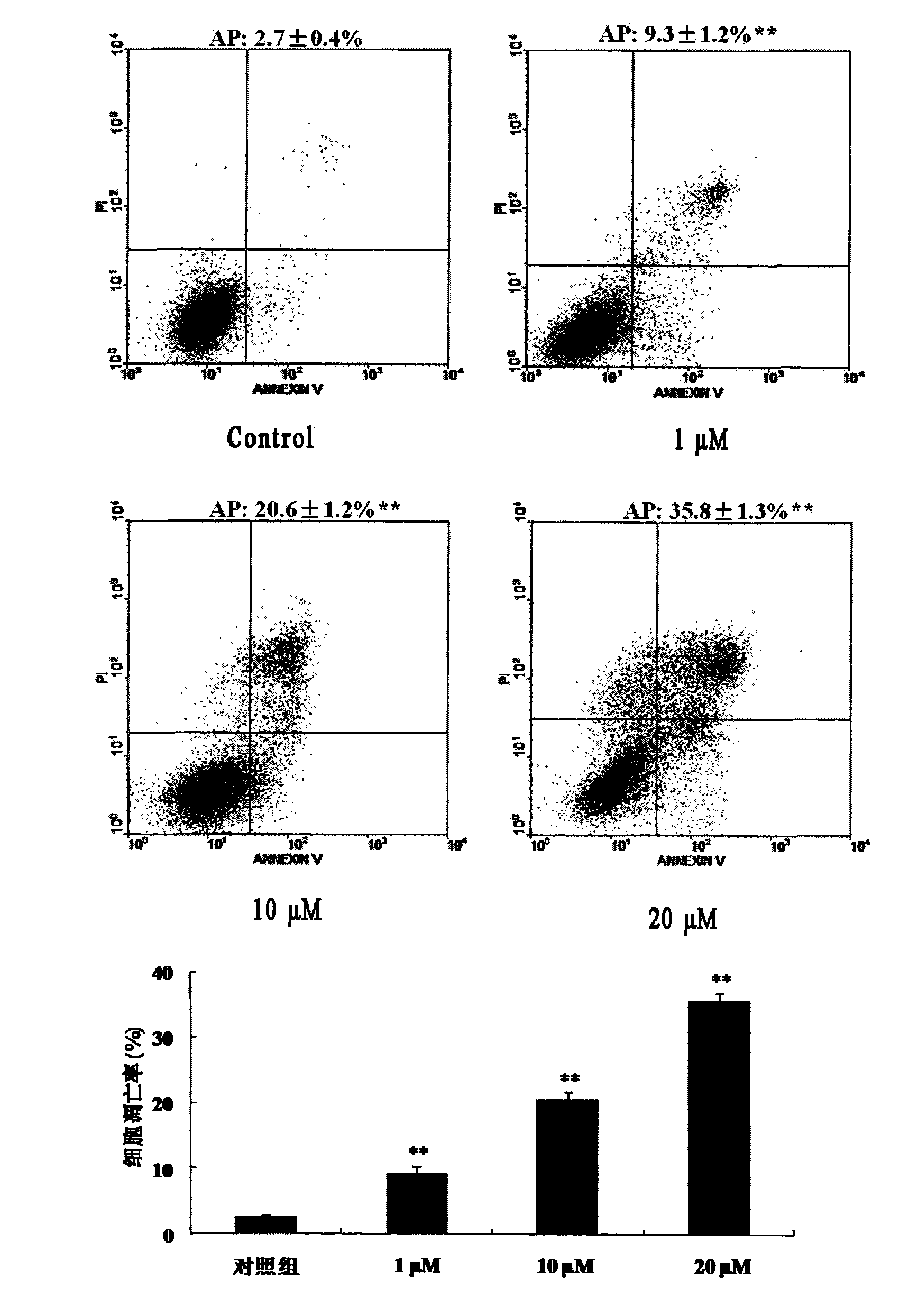
Application of Hyperforin derivant in preparing drugs
A technology of hypericin and hypericin ethyl ester is applied in the field of hypericin derivatives to achieve the effect of good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] One, the preparation method of the extract rich in hyperforin
[0031] (1) Take the above-ground part containing Hypericum perforatum (Hypericum perforatum L.), extract 4-10 times the dry weight of the medicinal material each time with a polar solvent containing 1-5% composite stabilizer at room temperature and stir for 2-6 hours , leaching 2-3 times; said polar solvent is alcohols (usually methanol, ethanol, propanol or isopropanol), ketones (usually acetone), ketone water mixed solvent or ketone alcohol water with any The mixed solvent of ratio; Said composite stabilizer is the mixture of VC or VC derivative and VE or VE derivative and organic acid, and the mixing ratio is VC or VC derivative: VE or VE derivative: organic Acid=20~200: 20~200: 1 (mass ratio); Said organic acid is usually any one in citric acid, benzoic acid, tartaric acid or their combination in any proportion;
[0032] (2) Concentrate the above obtained extract to a crude drug content of 0.1-1 g / ml, ...
Embodiment 1
[0045] Embodiment 1, the preparation that is rich in the extract of hyperforin
[0046] The Hypericum perforatum medicinal material is pulverized, and 300 g of medicinal material powder is taken, and 800 milliliters of acetone leaching containing 1% compound stabilizer (stabilizer is composed of VC: VE: citric acid=50: 100: 1) with 800 milliliters of acetone extraction is carried out altogether 3 times, each The next day, combine the acetone extracts, recover the acetone to 1000 ml, and then add 250 ml of distilled water. Extract with 1300 milliliters of petroleum ether, extract 2 times in total, combine petroleum ether phase and add vitamin E to the content of 1%. Concentrate to 600 ml and extract twice with 500 ml of 50% ethanol, and discard the ethanol phase. The petroleum ether phase was basified with 800 ml of 1% NaOH in 50% ethanol, and the petroleum ether phase was discarded. Use 10% hydrochloric acid to adjust the alkali-alcoholic solution to pH = 2, then extract 3 t...
Embodiment 2
[0047] Embodiment 2, the synthesis of hyperforin ethyl ester
[0048] The extract of hypericin perforatum was dissolved in pyridine solution, after it was completely dissolved, acetic anhydride was added to the solution, stirred and reacted in the dark at room temperature, dried, and after 24 hours of reaction, thin-layer chromatography showed that the reaction was relatively complete, and the raw material point was almost complete. disappear. Ice water was added to the reaction mixture to stop the reaction.
[0049] Add n-hexane-diethyl ether mixture (3:1) for extraction three times, combine the organic phases, and successively wash with saturated NaHCO 3 Wash with saturated brine. Anhydrous sodium sulfate was dehydrated overnight, the solid sodium sulfate was removed by filtration, and the filtrate was concentrated to dryness to obtain a brownish yellow solid. Through silica gel column chromatography (100-200 mesh), first elute with petroleum ether, and then elute with pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com