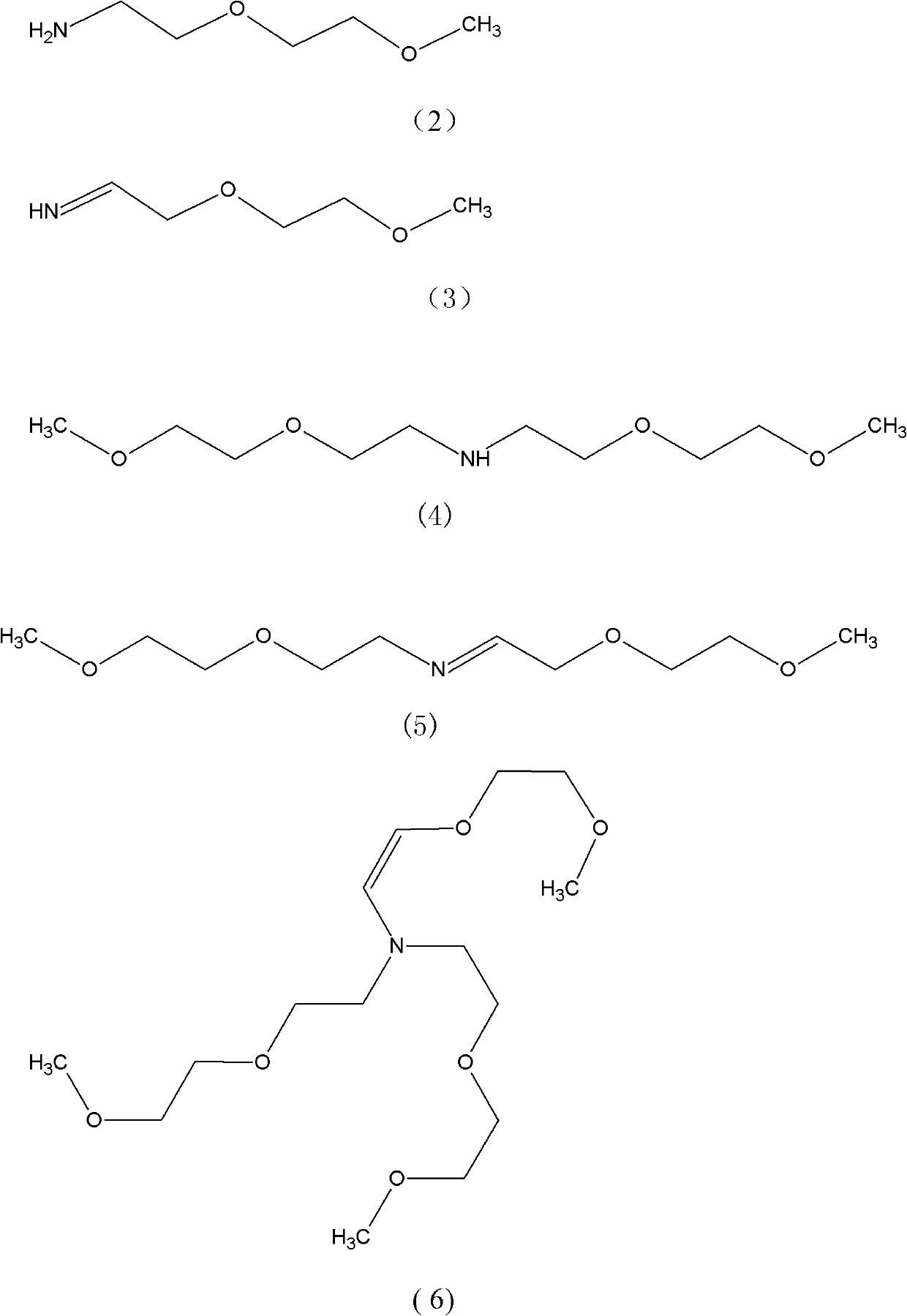
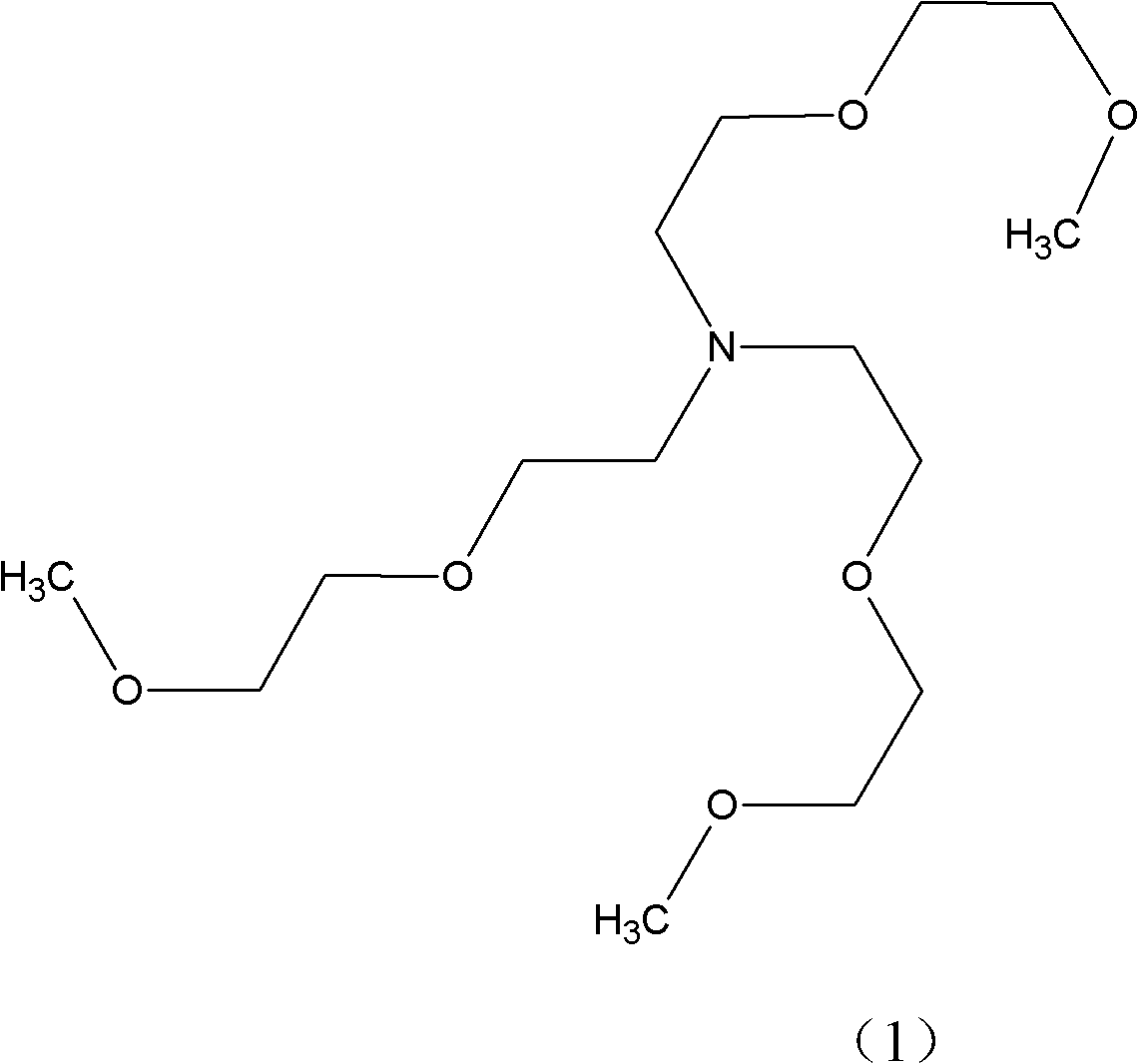
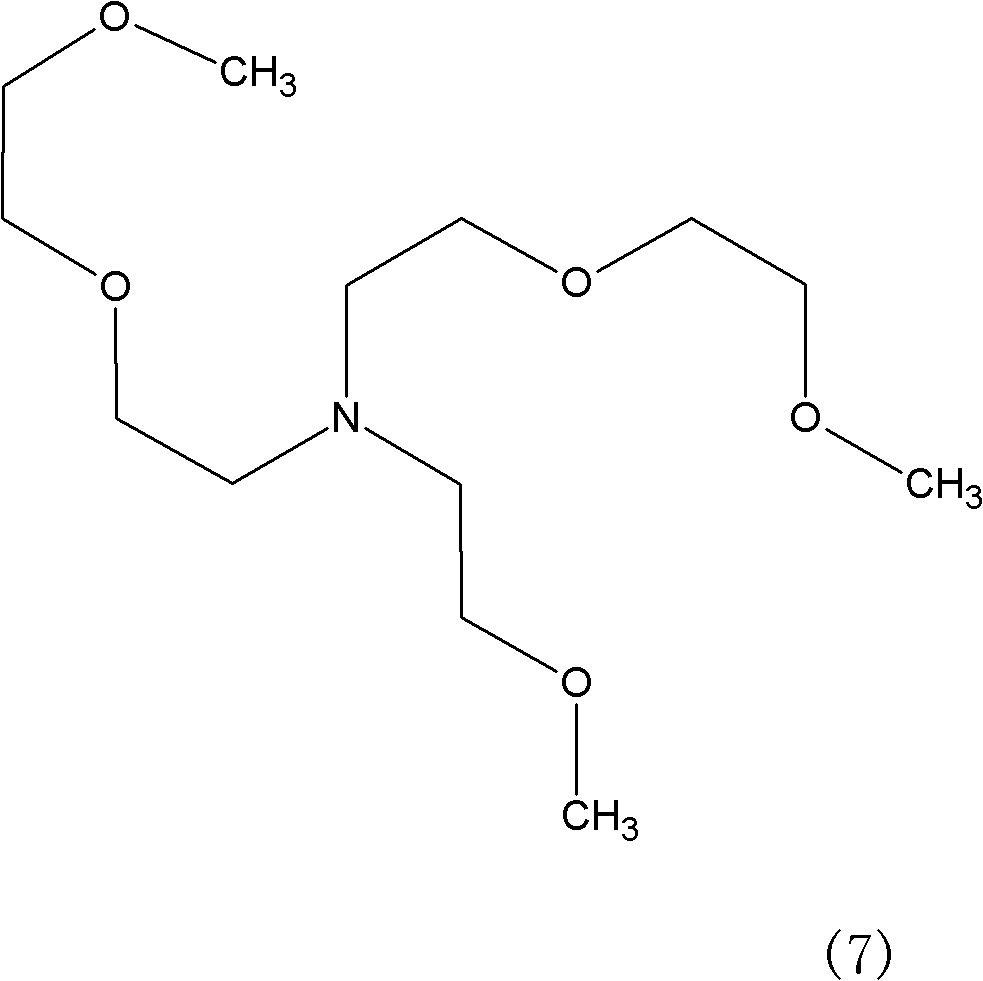
Method for synthesizing tris(dioxa-3,6-heptyl)amine
A technology for dioxeptyl and synthesis methods, which is applied in chemical instruments and methods, preparation of aminohydroxy compounds, preparation of organic compounds, etc., can solve the problems of poor selectivity and high reaction temperature, achieve high selectivity and reduce reaction temperature , improve the effect of selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] Preparation of skeleton Cu-Ni catalyst:
[0021] (1) 315kg of 20% sodium hydroxide solution is put into a 1000L reactor, the stirring and cooling water are turned on, the temperature is controlled at 30°C, and 100kg of alloy powder (Cu 12.5%, Ni 37.5%, Al 50%) is put into the reactor in batches. About 2 hours, then heat up to 90°C, react for 2 hours, add 500kg of water, cool down to 30°C, let stand for 20 minutes, suck the upper layer of water by vacuum, add 500kg of water to stir, let stand, remove water, until pH 7~8 , the skeleton catalyst of making skeleton Cu-Ni catalyst A 62.5kg, under the protection of water is put into barrel for standby, wherein Cu content is 20%.
[0022] (2) Repeat the above preparation method with 100kg of alloy powder (Cu 20.5%, Ni 29.5%, Al 50%) to obtain 68.3Kg of skeleton Cu-Ni catalyst B, wherein Cu content is 30%.
[0023] (3) Repeat the above-mentioned preparation method with 100kg alloy powder (Cu10.5%, Ni39.5%, Al50%), obtain skele...
Embodiment 1
[0024] Embodiment 1: Get 250kg Diethylene Glycol Monomethyl Ether (mass purity 99%), 50kg skeleton Cu-Ni catalyst A, drop into enamel kettle, stir and heat up dehydration, when the temperature in the kettle reaches 150 ℃, start to pass ammonia and hydrogen, pass Ammonia 8m 3 / hour, hydrogen flow 1m 3 / hour, excess ammonia was absorbed by a falling film absorption tower, and after ventilating for 5 hours, it was detected that the mass ratio of diethylene glycol monomethyl ether and 3,6-dioxaheptylamine in the reaction solution was 2.0:1.
[0025] Stop logical ammonia then, after continuing logical hydrogen 2.5 hours, detect and find in the reaction solution: Diethylene glycol monomethyl ether mass percentage drops to 1.8%, formula (7) mass percentage drops to 0.5%, formula (4) mass percentage drops to 1.2%, target product 95.2%, other 1.3%.
[0026] The catalyst was removed by filtration, and 205.26 kg of the product tris(3,6-dioxaheptyl)amine was obtained by rectification,...
Embodiment 2
[0027] Embodiment 2: Get 250Kg Diethylene Glycol Monomethyl Ether (mass purity 99%), 100kg framework Cu-Ni catalyst B, drop into enamel kettle, stir and heat up dehydration, when the temperature in the kettle reaches 130 ℃, start to pass ammonia and hydrogen, pass Ammonia 10m 3 / hour, hydrogen flow 1.1375m 3 / hour, excess ammonia was absorbed by a falling film absorption tower, ventilated for 4.0 hours, and it was detected that the mass ratio of diethylene glycol monomethyl ether and 3,6-dioxaheptylamine in the reaction solution was 1.9:1.
[0028] Then stop flowing ammonia, continue to pass hydrogen after 2 hours and 10 minutes, detect and find in the reaction solution: Diethylene glycol monomethyl ether mass percentage drops to 2.0%, formula (7) mass percentage drops to 0.4%, formula (4) mass percentage Down to 1.2%, target product 95%, other 1.4%.
[0029] The catalyst was filtered off, and 204.81 kg of tris(3,6-dioxaheptyl)amine was obtained by rectification, with a ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com