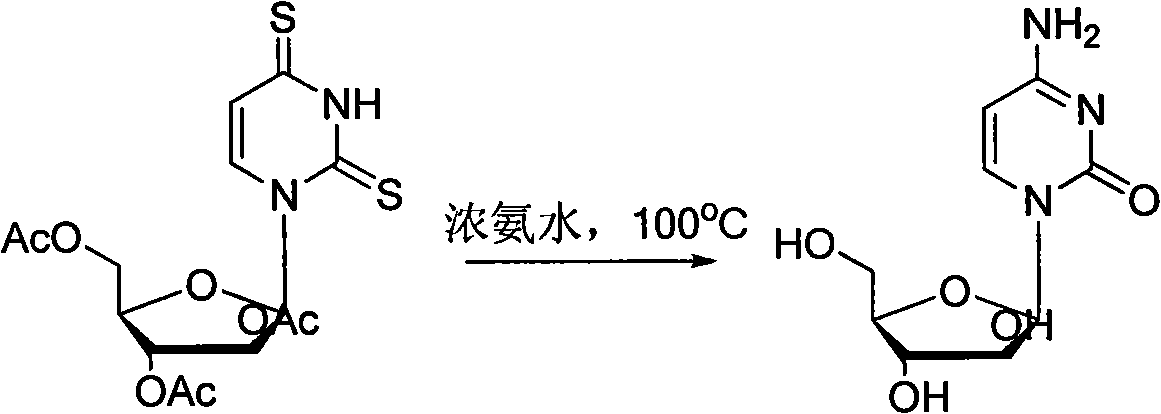
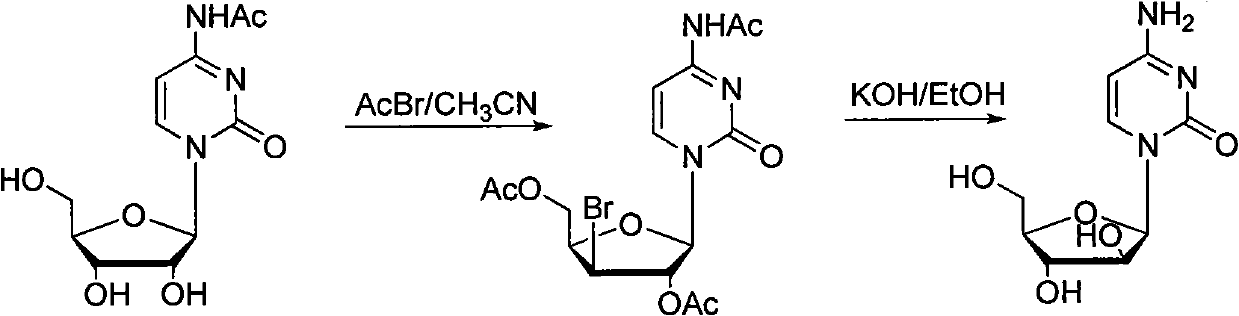
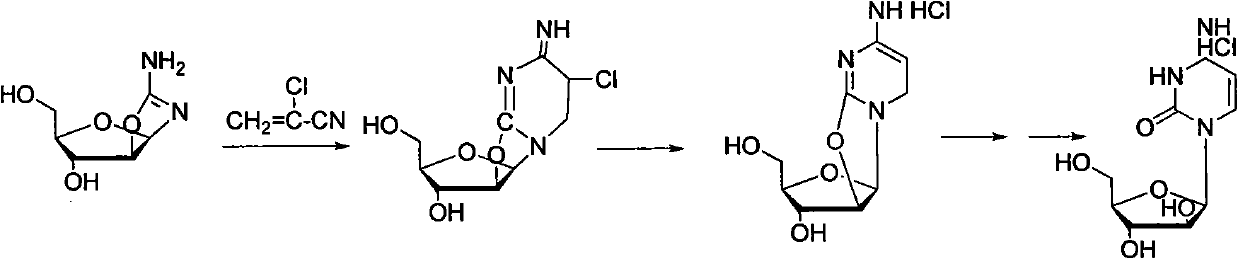
Technology for producing cytarabine through chemical synthesis method
A chemical synthesis method and cytarabine technology, which is applied in the field of synthesis technology of organic compounds, can solve the problems of high cost of cytarabine, many reaction steps, and many reagents involved, and achieves toxicity, easy recycling, and production. Low cost, stable and controllable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Add 80g (0.299mol) araburidine and 300mL hexamethyldisilazane HMDS into a 1L reactor, seal and pressurize (8Kg), heat slowly to 130°C, keep the temperature for 40h, then slowly cool down to about 60°C, Pour into a 1000mL three-neck flask, stir, concentrate under reduced pressure, recover excess hexamethyldisilazane HMDS, add a small amount of ethanol, concentrate to dryness, then add 500mL of methanol, cool down to about 10°C, and stir rapidly for 1 hour. Concentrate to dryness to obtain crude product. The crude product was recrystallized with acetone, filtered and dried to obtain 51 g of the product with a yield of 70.6%.
Embodiment 2
[0048] Add 80g (0.299mol) araburidine and 400mL hexamethyldisilazane HMDS into a 1L reactor, seal and pressurize (10Kg), heat slowly to 150°C, keep the temperature for 60h, then slowly cool down to about 80°C, Pour into a 1000mL three-necked flask, stir, concentrate under reduced pressure, recover excess hexamethyldisilazane HMDS, add a small amount of acetone, concentrate to dryness, then add 500mL methanol, cool down to about 20°C, and stir rapidly for 1 hour. Concentrate to dryness to obtain crude product. The crude product was recrystallized with ethanol, filtered, and dried to obtain 58 g of the product, with a yield of 80.5%.
Embodiment 3
[0050] Add 80g (0.299mol) araburidine and 500mL hexamethyldisilazane HMDS into a 1L reactor, seal and pressurize (10Kg), heat slowly to 150°C, keep the temperature for 72h, then slowly cool down to about 80°C, Pour into a 1000mL three-necked flask, stir, concentrate under reduced pressure, recover excess hexamethyldisilazane HMDS, add a small amount of methanol, concentrate to dryness, repeat three times, then add 500mL of methanol, cool down to about 20°C, and stir rapidly After 1 hour, concentrate to dryness to obtain crude product. The crude product was recrystallized with ethanol, filtered and dried to obtain 65 g of the product with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com