Application of syringin in preparation of medicine for treating acute gout
A technology for acute gout and syringin is applied in the application field of syringin in the preparation of medicines for treating acute gout, and can solve the problems of granulocytopenia, aplastic anemia and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 syringin preparation
[0040] Weigh 2 kg of Acanthopanax senticosus dry powder, reflux extraction with 70% ethanol for 3 times, the solid-liquid ratio is (1:8; 1:6; 1:5), 1h / time, the filtrates are combined, the ethanol is recovered, and the concentrated solution is added 8 times Amount of water was stirred to form a suspension, extracted three times with an equal amount of petroleum ether, and the aqueous layer was extracted four times with an equal volume of ethyl acetate. The ethyl acetate layers were combined, concentrated and dried under reduced pressure to obtain an extract. The extract was separated on a silica gel column and eluted sequentially with chloroform-methanol gradient with increasing polarity. When the ratio of chloroform-methanol was 100:4, crude crystals were eluted, and then recrystallized by methanol to obtain syringin crystals.
Embodiment 2
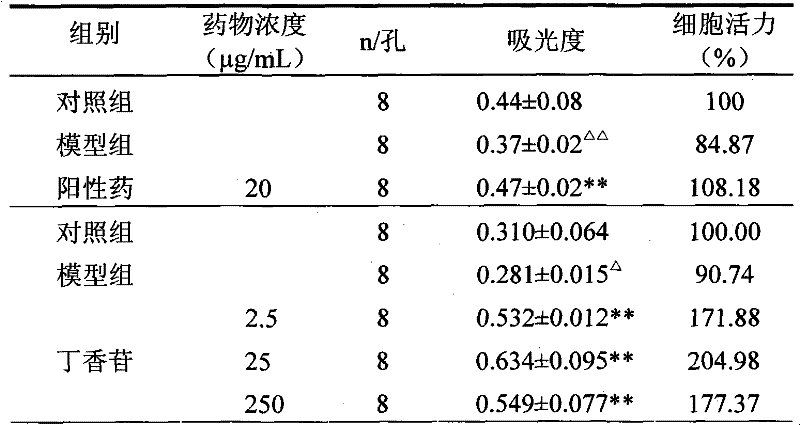
[0041] Example 2 Syringin protects HUVEC activity
[0042] 0.20 mg of syringin was dissolved in dimethyl sulfoxide (DMSO), the final concentration of DMSO was <0.02%, and serum-free DMEM culture solution was added to prepare a concentration of 20 μg / mL.
[0043] HUVEC were cultured in culture flasks, and when they grew to 70% to 80% confluence, they were digested with 0.25% trypsin, centrifuged, washed three times with 10% calf serum DMEM culture solution, and adjusted to 10% calf serum DMEM culture solution. 4×10 4 / mL cell suspension, implanted into a 96-well plate (200 μL per well), cultured for 24 hours, and gently sucked out the original culture solution, and performed the following experiments. Each group has 8 wells, and the specific grouping and liquid addition are as follows: control group (200 μL DMEM culture solution), model group (100 μg / mL MSU solution), syringin group (100 μg / mL MSU solution + 20 μg / ml syringin), add After liquid, continue to put 37 ℃, 5% CO 2...
Embodiment 3
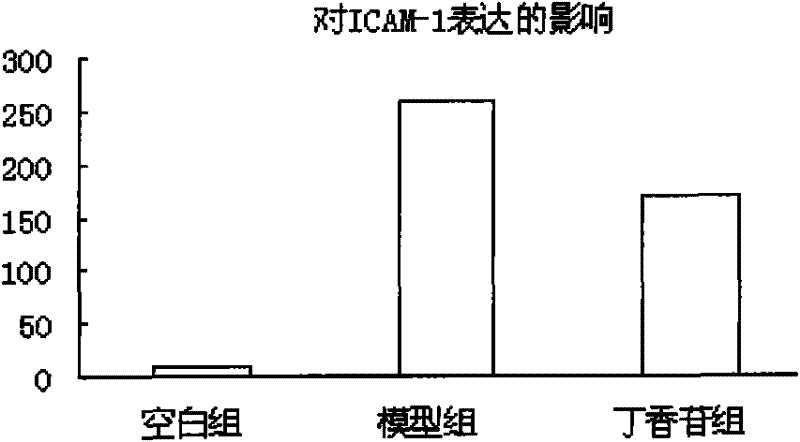
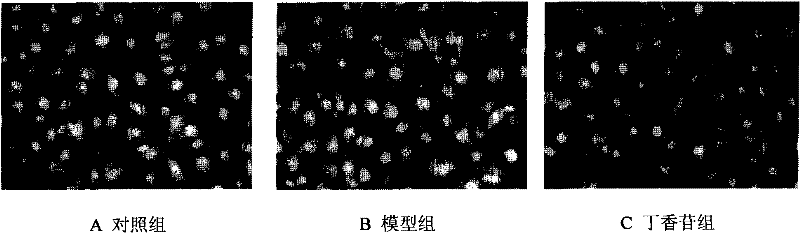
[0044] Example 3 Syringin inhibits ICAM-1 expression
[0045] Digest the HUVEC in the logarithmic growth phase with 0.25% trypsin, gently pipette to make a cell suspension, and adjust the cell density to 5×10 9 / L, inoculated in cell culture flasks. After the cells were congested (about 24 hours), the supernatant was discarded and divided into the following groups: control group, model group (100 μg / mL MSU solution), syringin group (100 μg / mL MSU solution + 20 μg / mL syringin), and continue After culturing for 24 hours, the cells were collected with PBS, centrifuged to remove the supernatant, and CD54 monoclonal antibody was added. After 30 min, the cells were washed with PBS, and the cells were resuspended. The percentage of positive cells was detected by flow cytometry (n=10000).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com