Hemostatic bacteriostatic biological dressing and preparation method thereof
A biological dressing and biological material technology, applied in the field of biomedical materials, can solve the problems affecting the antibacterial ability of biological sponges, the influence of nano-silver production, and limited penetration ability, so as to achieve improved hemostatic performance, good hemostatic performance, and antibacterial effect obvious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
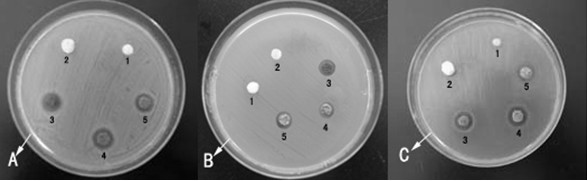
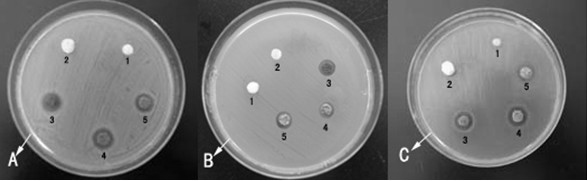
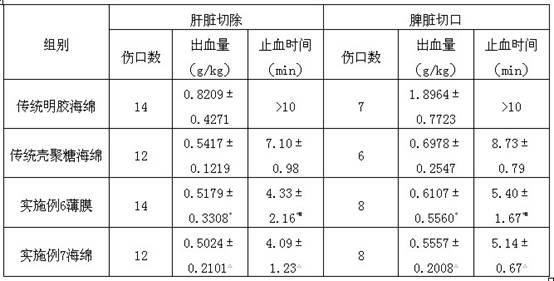
Image
Examples
Embodiment 1
[0030] Example 1 The biological dressing obtained in this embodiment is spongy, and chitosan is used as the biological dressing base material, the hemostatic drug adopts tranexamic acid, and the cross-linking agent adopts glutaraldehyde. The specific method is:
[0031] (1) Weigh 3 g of chitosan, dissolve it in an acetic acid solution with a concentration of 3% by volume, and make a colloidal liquid with a mass fraction of 3%, and add glutaraldehyde whose weight is 0.01% of the weight of the colloidal liquid, Stir evenly, place at 4°C for 4 hours, then pour into a rectangular container, freeze-dry to form a biological sponge, and finally heat and solidify the biological sponge at 80°C for 5 hours;
[0032] (2) Immerse the biological sponge obtained above in a silver nitrate solution with a concentration of 0.1 mM, place it in a sterilizer at a high temperature of 121°C and a high pressure of 15 psi for 10 minutes, then take it out and rinse it with deionized water to obtain ...
Embodiment 2
[0035] Example 2 The biological dressing obtained in this embodiment is film-like, and is made into the biological dressing matrix with carboxymethyl chitosan, and the hemostatic drug adopts thrombin. The specific method is:
[0036] (1) Weigh 5g of carboxymethyl chitosan, dissolve it in deionized water, and make a colloidal liquid with a mass fraction of 5%, add silver nitrate solution to this colloidal liquid, stir evenly, and then heat the and 15 psi high pressure, place it in a sterilizer for 10 minutes, take it out to get the biological glue containing nano-silver;
[0037](2) Add 5 mg of thrombin to the biological glue containing nano-silver, stir evenly, pour it on a glass or stainless steel plate, cast it into a film, and dry it at room temperature to obtain a film-like hemostatic and antibacterial biological dressing.
[0038] In the film-like hemostatic and antibacterial biological dressing obtained above, the weight of nano-silver is 0.01% of the weight of the bi...
Embodiment 3
[0039] Example 3: The biological dressing obtained in this example is in the shape of a sponge, and chitosan and sodium alginate are used as the biological dressing matrix, the hemostatic drug is tranexamic acid, and the cross-linking agent is genipin. The specific method is:
[0040] (1) Weigh 5g of chitosan and 3g of sodium alginate, dissolve in acetic acid solution with a concentration of 3% by volume, and make a colloidal liquid with a mass fraction of 1%, and add 0.3% of the weight of the colloidal solution Genipin aqueous solution, stirred evenly, placed at 4°C for 4 hours, then poured into a rectangular container, freeze-dried to form a biological sponge, and finally heated and solidified the biological sponge at 100°C for 4 hours;
[0041] (2) Immerse the biological sponge obtained above into a silver nitrate solution with a concentration of 0.25 mM, place it in a sterilizer at a high temperature of 121°C and a high pressure of 15 psi for 15 minutes, then take it out...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com