Method for preparing 1,2,3,4,6-penta-O-galloyl-beta-D-glucose (PGG)
A technology of galloyl and glucose, applied in the field of preparation of 1,2,3,4,6-penta-O-galloyl-β-D-glucose, which can solve the cumbersome separation and purification process, low yield, high cost, etc. problem, to achieve the effect of no solvent residue, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
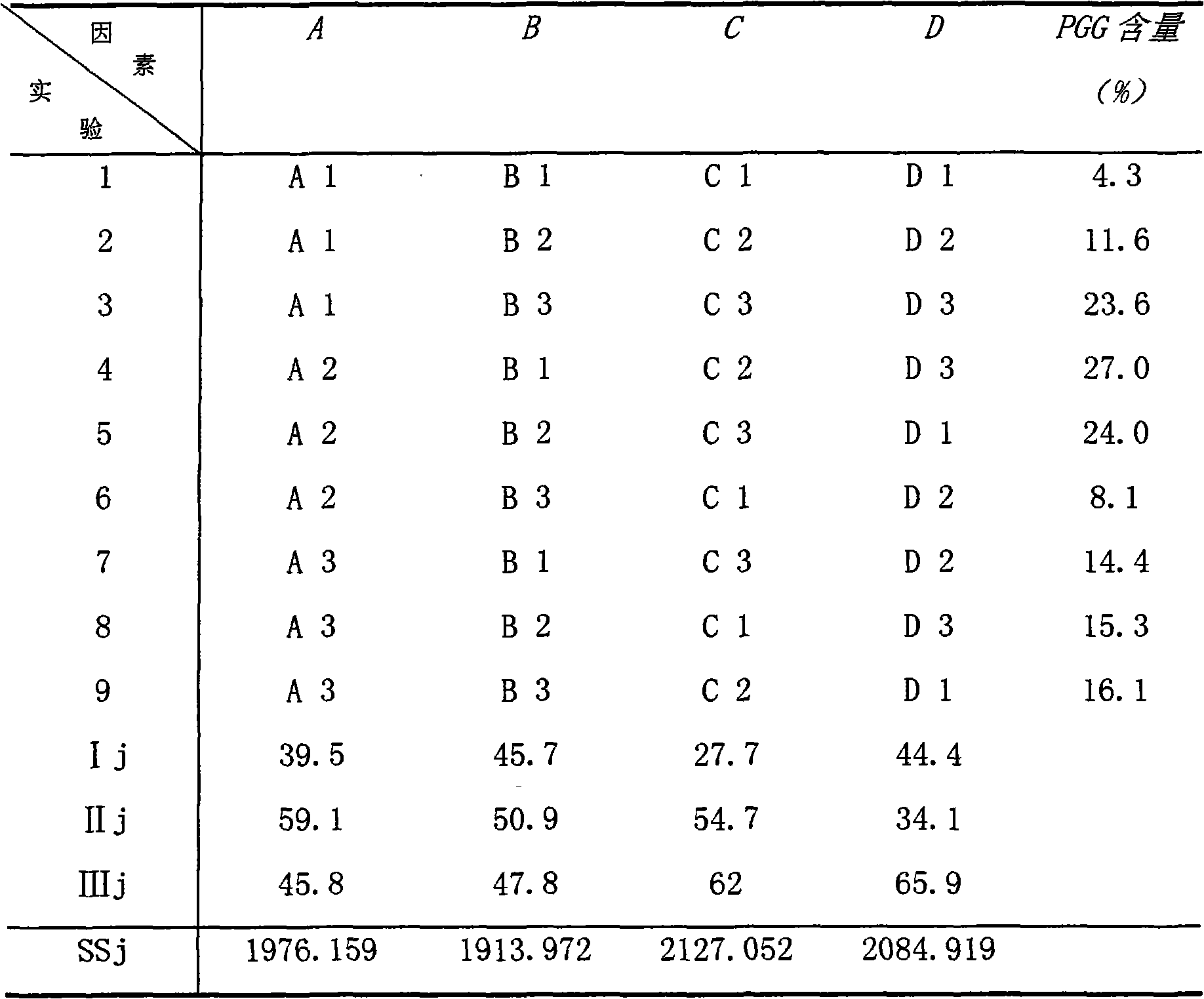
[0039] The specific steps for the preparation of 1,2,3,4,6-penta-O-galloyl-β-D-glucose and its activity determination in the present invention are as follows:
[0040] (1) Dissolve tannic acid in methanol-water solution containing HAc-NaAc buffer solution (PH=6), and hydrolyze in a water bath at 50° C. for 12 hours in a sealed manner.
[0041] (2) The above hydrolyzate was evaporated to dryness under reduced pressure at 48°C, dissolved in methanol, and then slowly poured into the upper end of a Sephadex column (such as: Sephadex LH-20, etc.) chromatography column. About 100g of tannic acid is loaded on a 41×6.4cm column material. Use methanol for elution, and the elution flow rate is controlled at about 2 mL / min. The eluate enriched in 1,2,3,4,6-penta-O-galloyl-β-D-glucose was collected, concentrated, and dried to obtain crude PGG, whose purity was greater than 85% as determined by HPLC.
[0042] (3) Dissolve the above-mentioned 1,2,3,4,6-penta-O-galloyl-β-D-glucose crude pr...
Embodiment 2
[0065] (1) Weigh 90g of industrial tannic acid, prepare a solution with 135mL of HAc-NaAc buffer solution with pH=6.0, and 1.2L of methanol, and hydrolyze it in a water bath at 50°C for 12 hours.
[0066] (2) The hydrolyzate was evaporated to dryness under reduced pressure at 48°C, dissolved in a small volume of methanol, and then slowly poured into the upper end of a Sephadex LH-20 chromatography column. Use methanol for elution, and the elution flow rate is controlled at about 2 mL / min. The eluate enriched in 1,2,3,4,6-penta-O-galloyl-β-D-glucose was collected, concentrated, and dried to obtain crude PGG, whose purity was greater than 85% as determined by HPLC.
[0067] (3) Dissolve the above-mentioned 1,2,3,4,6-penta-O-galloyl-β-D-glucose crude product in a small volume of aqueous solution and slowly pour it into a macroporous adsorption resin (such as: Diaion HP20, D- 101, KB-8, etc.) the upper end of the chromatography column. First, gradient elution with water, 10%, 20...
Embodiment 3
[0072] Tablet: 10 mg of the compound 1,2,3,4,6-penta-O-galloyl-β-D-glucose obtained in Example 2, 180 mg of lactose, 55 mg of starch, and 5 mg of magnesium stearate;
[0073] Preparation method: mix the compound, lactose and starch, moisten it evenly with water, sieve the wetted mixture and dry it, then sieve it, add magnesium stearate, then press the mixture into tablets, each tablet weighs 250mg, and the compound content is 10mg .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com