Phenolic compound in tobaccos and preparation method and application thereof
A compound and tobacco technology, applied in the field of tobacco chemistry, can solve problems such as components that have not been identified, and achieve the effect of accelerating aging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] - Compound preparation
[0027] Tobacco samples were collected in Yuxi, Yunnan, and the variety was Honghua Dajinyuan. Sampling 2.5kg of the tobacco sample was pulverized into 30 meshes, extracted 3 times with 70% ethanol by ultrasonic, the extracts were combined and concentrated into an extract, and 212g of extract was obtained. After dissolving the extract with an appropriate amount of acetone, mix the sample with 300g of crude silica gel (80-100 mesh), pack 2.5kg of silica gel (160-200 mesh) into a column for silica gel column chromatography, and use a gradient of chloroform:acetone (1:0→0:1) Elution, TLC monitoring combined the same fractions to obtain 8 fractions (pure chloroform, chloroform-acetone 20:1, chloroform-acetone 9:1, chloroform-acetone 8:2, chloroform-acetone 3:2, chloroform-acetone 1 : 1, chloroform-acetone 1: 2, pure acetone), wherein 15.4 g of chloroform-acetone (1: 1) eluted part is separated by Agilent 1100 semi-preparative high performance liquid...
Embodiment 2
[0029] - identification of compounds
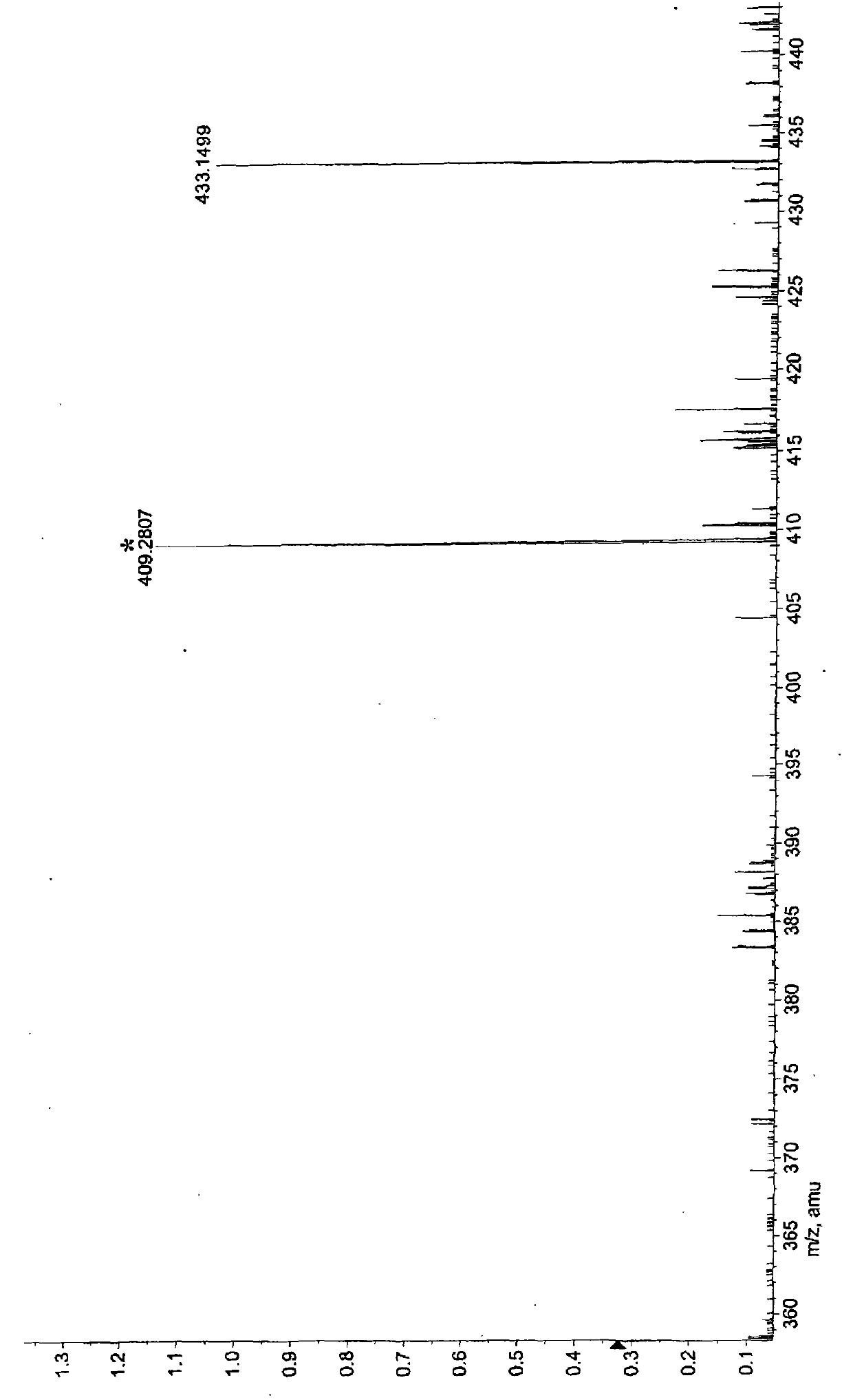
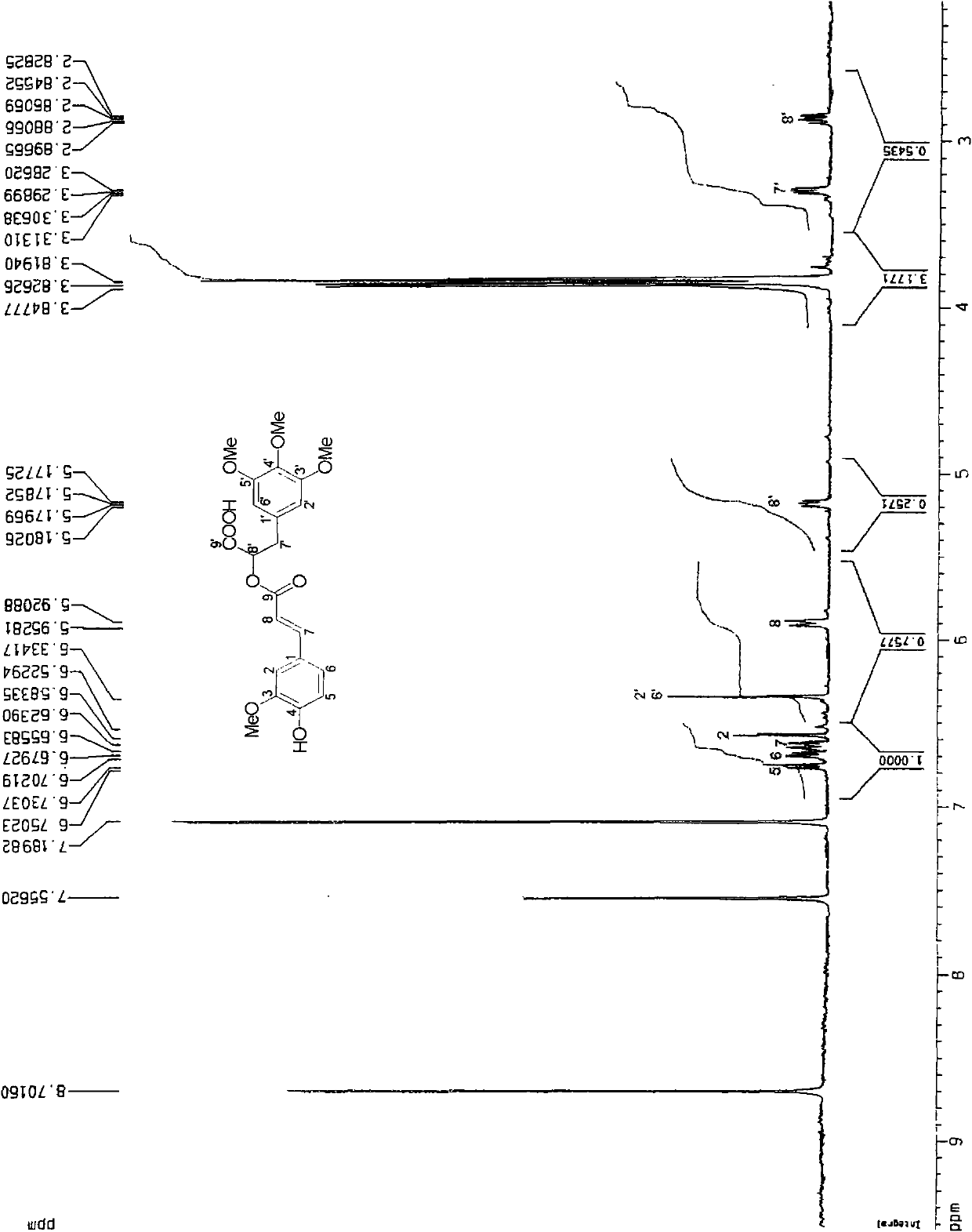
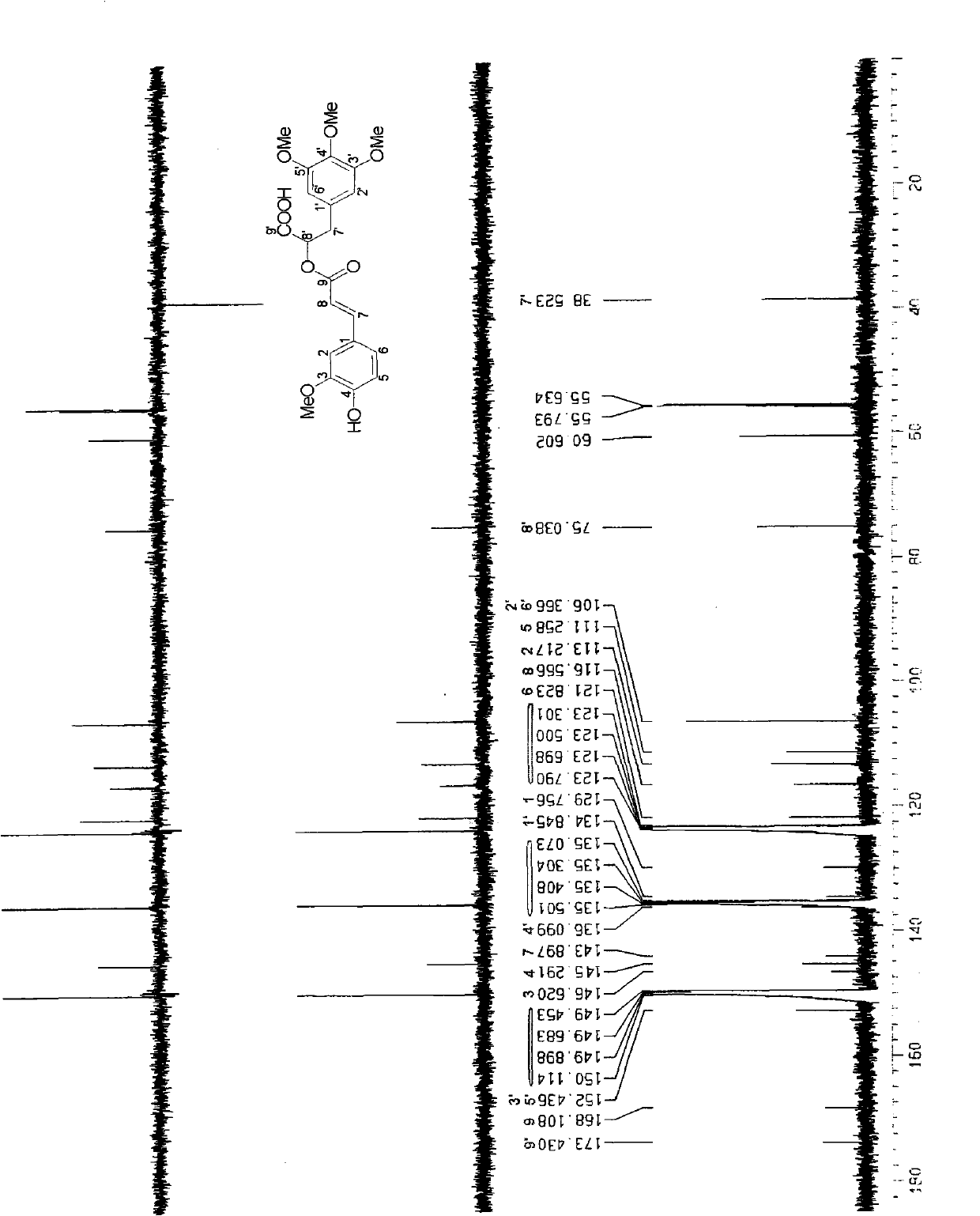
[0030] This patent compound is light yellow powder; Ultraviolet spectrum (solvent is methanol), λ max (logε)278(3.12), 238(3.31), 210(3.97)nm; infrared spectrum (potassium bromide tablet) v max 3490, 3024, 2897, 2852, 1708, 1655, 1632, 1590, 1450, 1392, 1154, 1038, 918, 848cm -1 ; HRESIMS (Figure-1) gives quasi-molecular ion peak m / z 433.1499[M+H] + (calculated value 433.1492). combine 1 H and 13 C NMR spectrum gives a molecular formula C 22 h 24 o 9 , with an unsaturation of 11. From 1 H and 13 CNMR spectrum (Figure-2 and Figure-3) signals can be seen that there are 2 benzene rings in the compound (including 5 double-bonded methine carbons, δ C 106.3, 106.3, 111.3, 113.2, 121.8); in addition, there is an aliphatic carbonyl (δ C 168.1), a carboxylic acid carbonyl (δ C 173.4), 1 methine (δ C38.5 ), 1 oxidized methylene (δ C 75.0), a group of double bonds ((δ C 143.9, 116.5), 4 methoxy groups (δ C 55.6, 55.6, 55.8, 60.6). ...
Embodiment 3
[0032] ——Detection of compound antioxidant activity
[0033] The antioxidant activity is expressed by the ability to scavenge DPPH free radicals; the activity of scavenging lipid free radicals DPPH is determined with 50 μg / mL as the initial screening concentration. Take a costar 96-well plate and add freshly prepared DPPH ethanol solution (6.5×10 5 mol / L) 190 μL / well, add 10 μL / well of the sample to be tested, add 10 μL of normal saline to the blank well, mix well, seal the plate with a sealing film, keep it at room temperature for 30 minutes in the dark, and measure it on a UV2401 spectrophotometer Measure the absorbance value of each hole on the instrument, and the measurement wavelength is 517nm; the scavenging rate of the sample to the lipid free radical DPPH is calculated according to the following formula:
[0034] DPPH clearance rate (%) = (A 空白 -A 样品 ) / A 空白 ×100%
[0035] A 空白 : Absorbance value of the blank control group; A 样品 : Add the absorbance value of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com