Escherichia coli TolC antigen as well as antibody and application thereof
A technology of Escherichia coli and antigens, applied in the fields of application, antibodies, antibacterial drugs, etc., can solve the problems of unclear side reactions and target sequence specificity, and achieve the function of inhibiting drug resistance, overcoming antibody heterophile, and using safe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
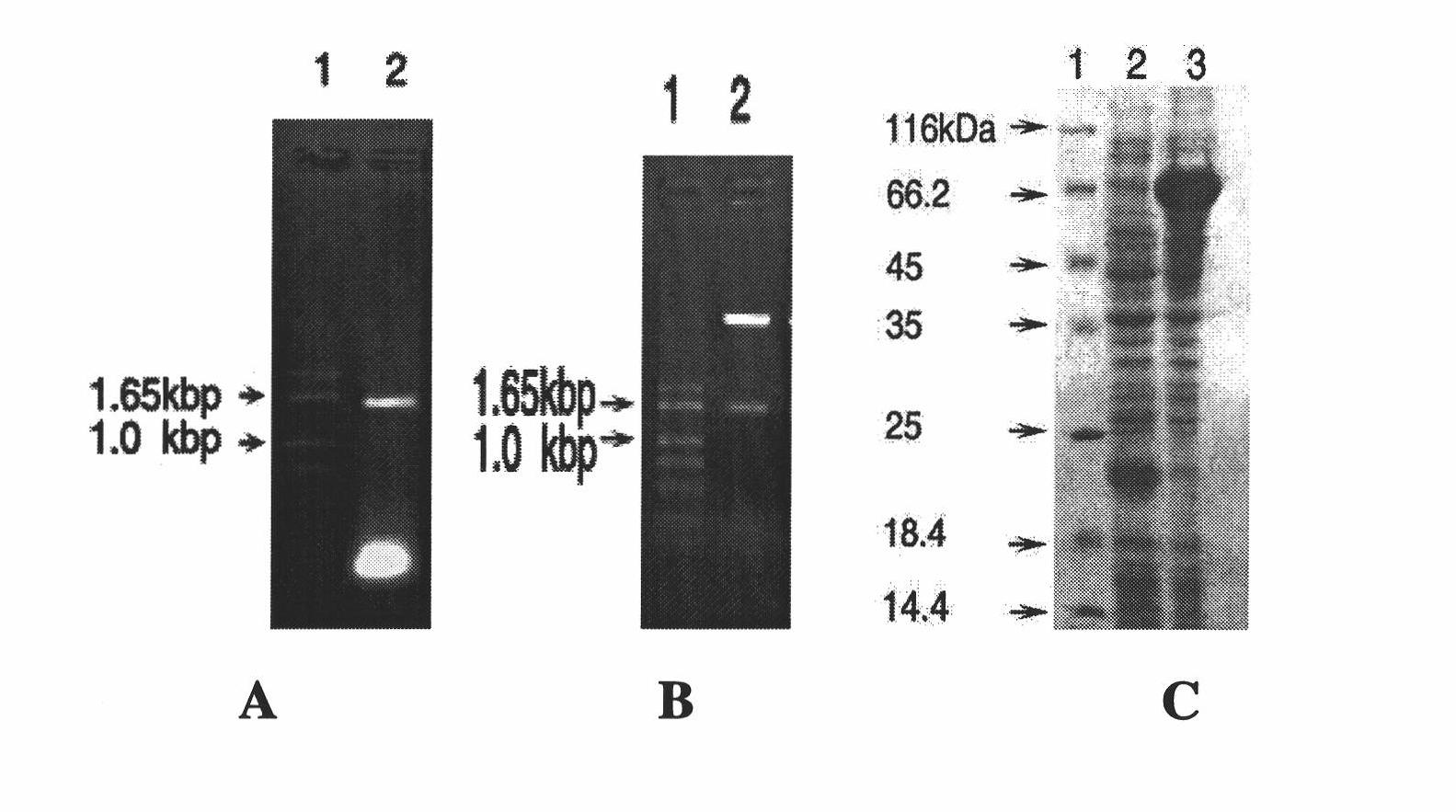
[0027] TolC gene cloning, prokaryotic expression, protein purification and preparation of rabbit antiserum
[0028] In this embodiment, the full-length E. coli TolC coding sequence or fragment is constructed into the E. coli protein prokaryotic expression vector to achieve expression and purification of the target protein.
[0029] The coding sequence of the TolC gene is shown in SEQ ID NO:3.
[0030] SEQ ID NO: 3:
[0031] 1 atgaagaaat tgctccccat tcttatcggc ctgagccttt ctgggttcag ttcgttgagc
[0032] 61 caggccgaga acctgatgca agtttatcag caagcacgcc ttagtaaccc ggaattgcgt
[0033] 121 aagtctgccg ccgatcgtga tgctgccttt gaaaaaatta atgaagcgcg cagtccatta
[0034] 181 ctgccacagc taggtttagg tgcagattac acctatagca acggctaccg cgacgcgaac
[0035] 241 ggcatcaact ctaacgcgac cagtgcgtcc ttgcagttaa ctcaatccat ttttgatatg
[0036] 301 tcgaaatggc gtgcgttaac gctgcaggaa aaagcagcag ggattcagga cgtcacgtat
[0037] 361 cagaccgatc agcaaacctt gatcctcaac accgcgaccg cttatttcaa cgtgttgaat
[0038] 421 gctattgacg ttctttccta ta...
Embodiment 2
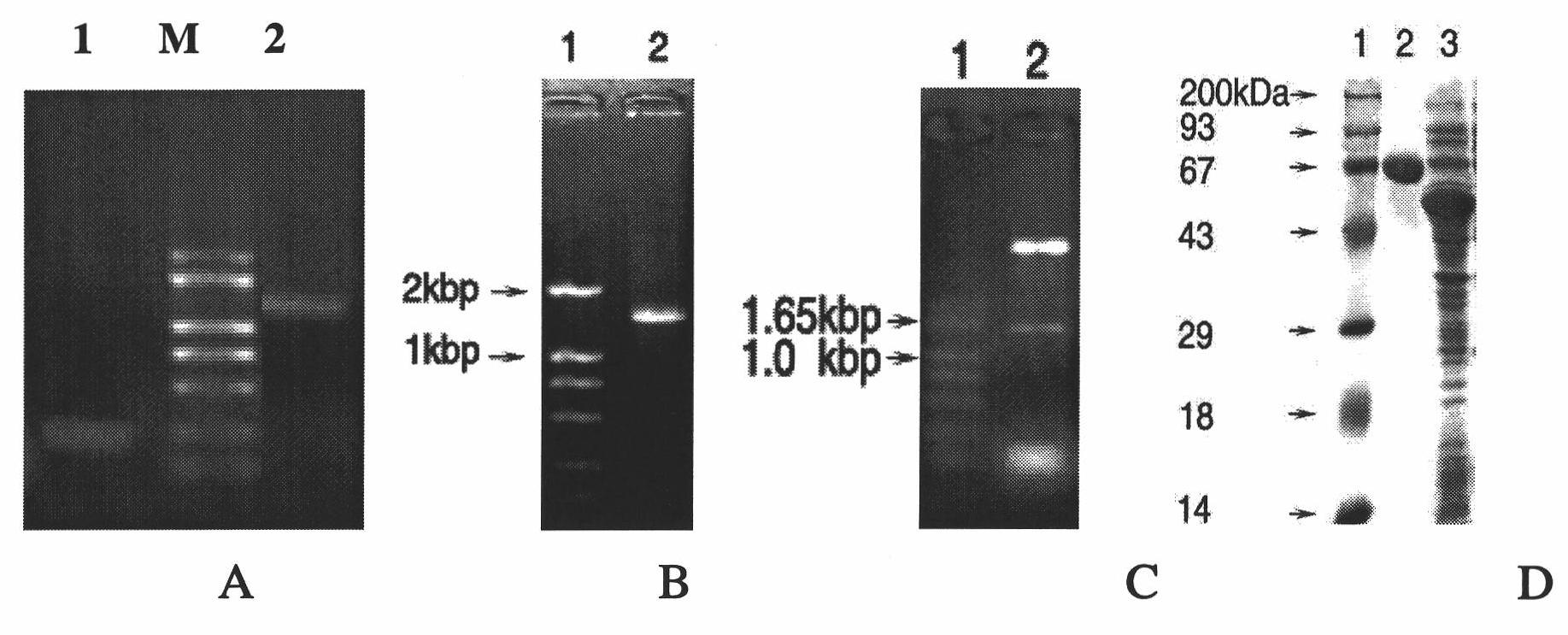
[0077] Cloning of TolC fragment SNGYRDANGI mutation gene (tolC1)
[0078] 1. Design of primers for TolC fragment mutant gene tolC1
[0079] Based on the principle of replacing hydrophobic amino acids with other hydrophobic amino acids and replacing hydrophilic amino acids with other hydrophilic amino acids, the fragment SNGYRDANGI in the TolC amino acid sequence was replaced with amino acids, and the replacement result was: RQCATKFHCL. In the nucleotide sequence of the tolC gene, find the nucleotide sequence corresponding to the amino acid sequence (SEQ ID NO: 7): 5'-AGC AAC GGC TAC CGC GAC GCG AACGGC ATC-3', according to the amino acid codon, The nucleotide sequence was correspondingly replaced with (SEQ ID NO: 8): 5'-CGT CAG TGC GCT ACT AAG TTC CAT TGC CTG-3'. Therefore, according to the nucleotide sequence of the tolC gene, the short-segment mutation tolC gene (named tolC1) was designed with two pairs of primers at the 5'end and the 3'end (Table 1), and at the same time, the 5'...
Embodiment 3
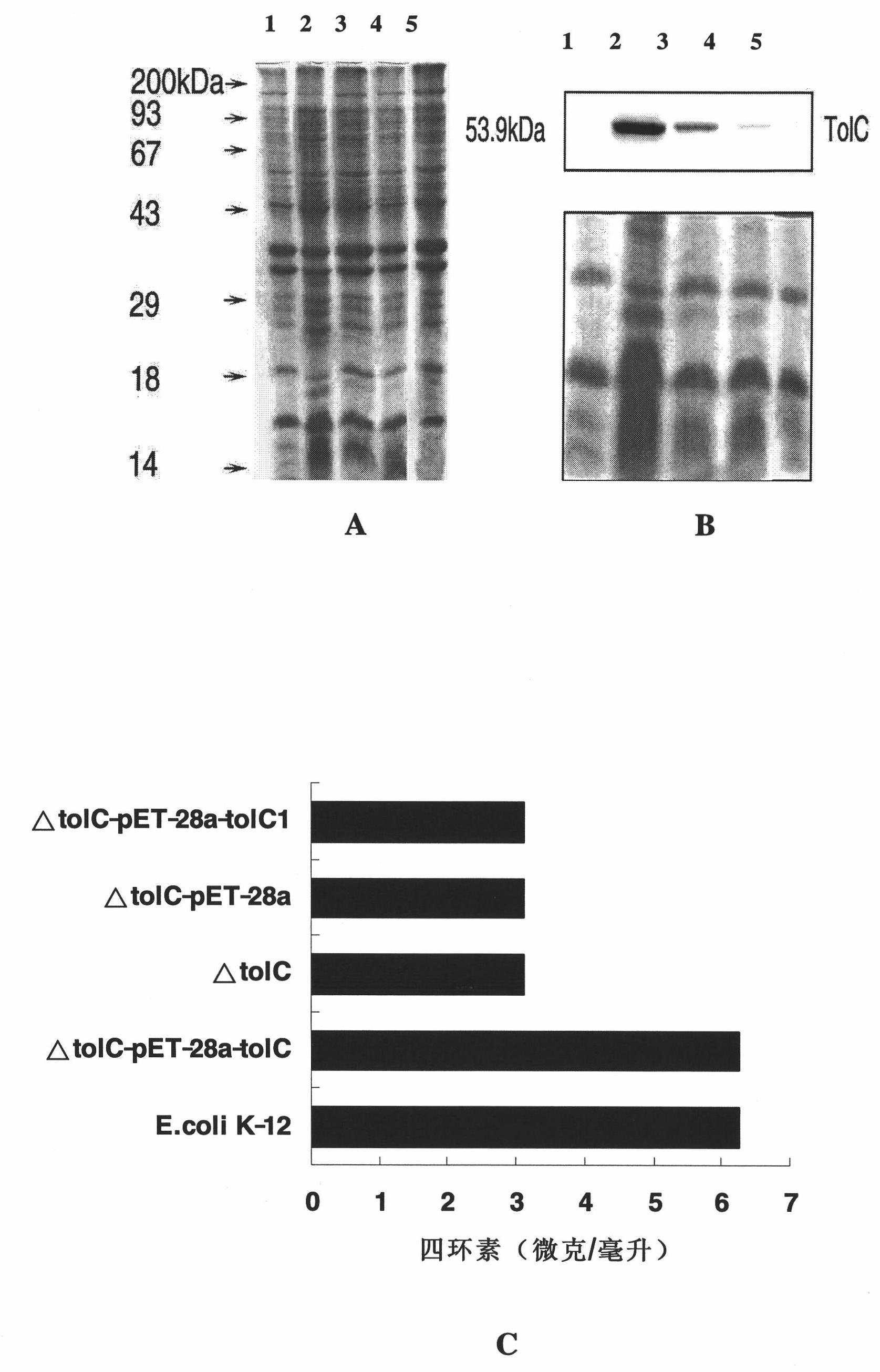
[0093] Construction process of Escherichia coli tolC gene deletion strain and corresponding other strains
[0094] 1. Construction process of E. coli tolC gene deletion strain:
[0095] First, a competent strain of BW25113 strain containing pKD46 plasmid (gifted by Professor Barry L. Wanner of Purdue University) was prepared. Inoculate the BW25113 strain containing pKD46 plasmid in 5mL LB medium, cultivate overnight at 30℃, inoculate 100mL LB medium containing 70mg / mL ampicillin at 1:50 the next day, and when cultivated at 30℃ to 0.25 (OD600), add L -Arabinose to 30mmol / L, induce 1 hour (OD600 can not exceed 0.6), so that the Red recombinase (Exo, Bet and Gam protein) on the pKD46 plasmid can be fully expressed. After pre-cooling on ice for 10 minutes, the precipitate was collected by centrifugation at 4°C, washed with pre-cooled 10% glycerol 3 times, concentrated 100 times into 1 mL competent cells, and stored in a -80°C refrigerator for later use.
[0096] According to the TolC g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com