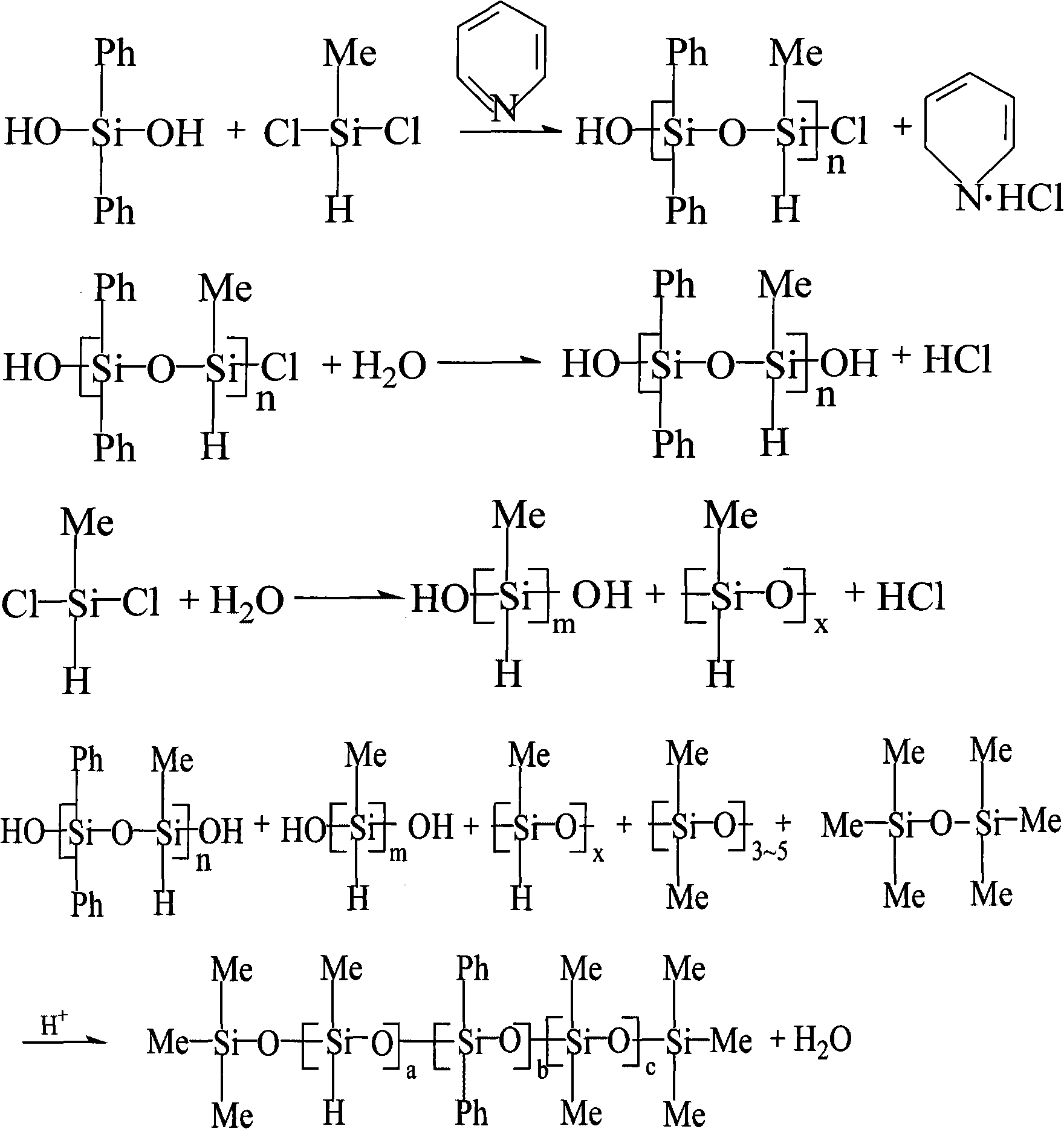Synthesis method of phenyl hydrogen-containing silicone oil
A technology of phenyl hydrogen-containing silicone oil and synthesis method, which is applied in the field of synthesis of organic polymer compounds, can solve problems such as difficult process control, high cost, and serious pollution, and achieve simple and easy process operation, good transparency, and high refraction rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 432g of diphenyldihydroxysilane into a 3000ml three-necked flask containing 1500g of toluene and 320g of pyridine, and slowly drop into a mixed solution of 230g of methylhydrogendichlorosilane and 300g of toluene under vigorous stirring, and the dropping temperature is controlled at 25°C the following. After the dropwise addition was completed, stirring was maintained at room temperature for 60 min. Then add 40°C deionized water, and wash the organic layer repeatedly until neutral. The organic layer was taken, and 201 g of D4, 39 g of hexamethyldisiloxane and 37.8 g of concentrated sulfuric acid were added to react at room temperature for 4 hours. Then stand for stratification, remove sulfuric acid, then add 1000g of 3% sodium bicarbonate solution and stir for 15min, remove the water layer, and then repeatedly wash the organic layer with 40°C deionized water until neutral. After the organic layer was filtered, the solvent was removed by heating, and the low molecu...
Embodiment 2
[0027] Add 432g of diphenyldihydroxysilane into a 3000ml three-necked flask containing 1500g of toluene and 320g of pyridine, and slowly drop into a mixed solution of 230g of methylhydrogendichlorosilane and 300g of toluene under vigorous stirring, and the dropping temperature is controlled at 25°C the following. After the dropwise addition was completed, stirring was maintained at room temperature for 60 min. Then add deionized water at 50°C, and wash the organic layer repeatedly until neutral. The organic layer was taken, and 148g of DMC (dimethylsiloxane mixed ring, D3-D5), 35.9g of hexamethyldisiloxane and 35g of concentrated sulfuric acid were added to react at room temperature for 4 hours. Then stand for stratification, remove sulfuric acid, add 1000g of 3% sodium bicarbonate solution and stir for 15min, remove the water layer, and then repeatedly wash the organic layer with deionized water at 50°C until neutral. After the organic layer was filtered, the solvent was re...
Embodiment 3
[0029] Add 432g of diphenyldihydroxysilane into a 3000ml three-necked flask containing 1500g of toluene and 320g of pyridine, and slowly drop into the mixed solution of 460g of methylhydrogendichlorosilane and 300g of toluene under vigorous stirring, and the dropping temperature is controlled at 25°C the following. After the dropwise addition was completed, stirring was maintained at room temperature for 60 min. Then add deionized water at 60°C, and wash the organic layer repeatedly until neutral. The organic layer was taken, and 33g of D4, 36.1g of hexamethyldisiloxane and 48g of concentrated sulfuric acid were added to react at room temperature for 4 hours. Then stand for stratification, remove sulfuric acid, then add 1000g of 3% sodium bicarbonate solution and stir for 15min, remove the water layer, and then repeatedly wash the organic layer with deionized water at 60°C until neutral. After the organic layer was filtered, the solvent was removed by heating, and the low mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com