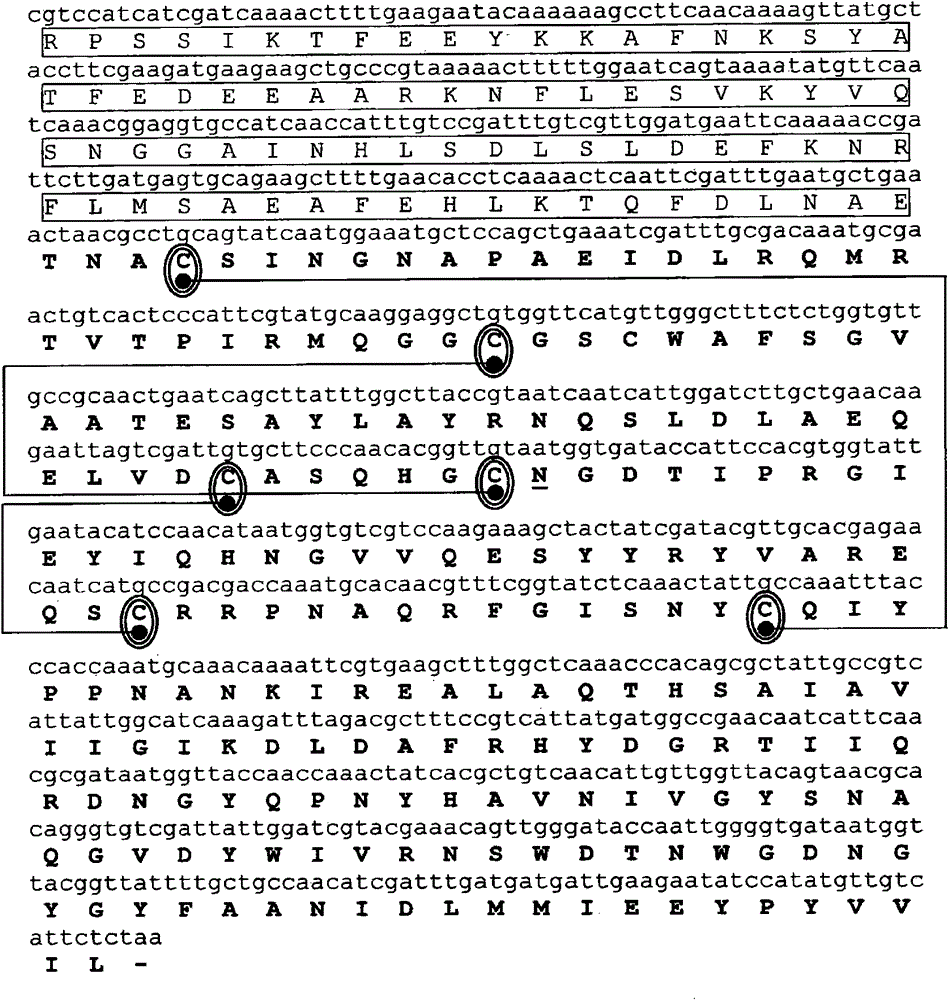
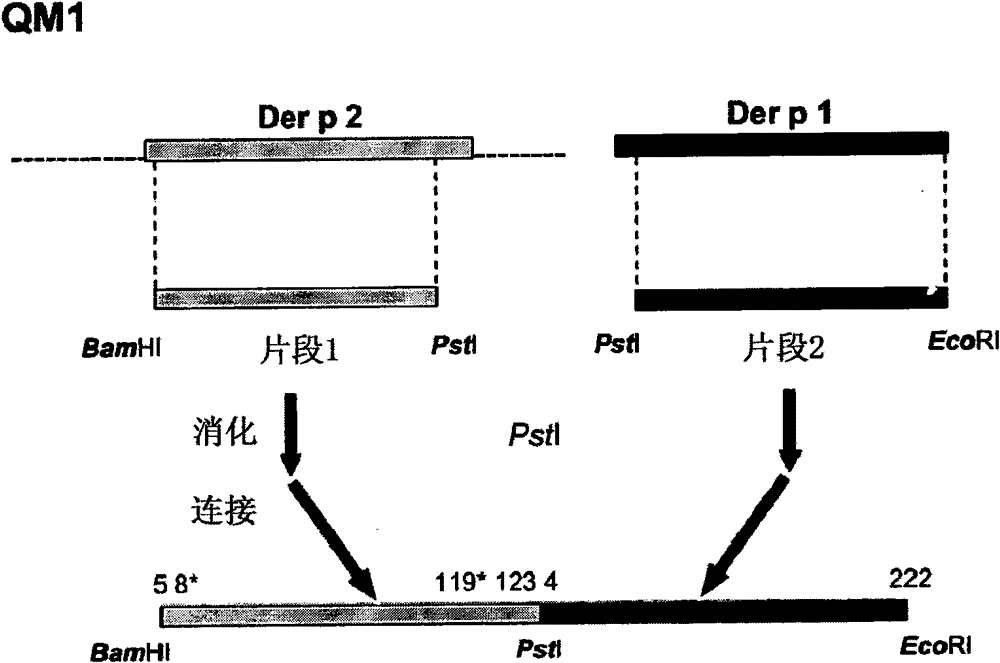
Hypoallergenic hybrid proteins of major class 1 and class 2 mite allergens for the treatment of allergy
A technology of host cells and sequences, applied in the directions of allergen antigen components, gene therapy, fusion polypeptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0194] Example 1: Purification of natural allergens Der p 1 and Der p 2 from mite bodies
[0195] Using a mixture of freeze-dried body and feces of D. dust mite (Laboratorios Leti, Madrid, Spain) as starting material, 10 volumes (p / v) of PBS (phosphate-buffered saline) supplemented with 1 mm PMSF (phenylmethylsulfonyl fluoride) brine) at 4°C for 15 minutes with rapid agitation. Then centrifuge at 3,800 xg for 15 minutes at 4°C. The extraction supernatant was filtered through AP (Millipore) and 60% ammonium sulfate (361 g / l) was added slowly for 30 min. After stirring for 1 hour at 4°C, it was centrifuged at 17,000 xg for 15 minutes at 4°C.
[0196] ·Purification of native Der p 1
[0197] The pellet obtained after centrifugation was resuspended in 2 ml 20 mM Tris pH 8.0 and filtered through 0.22 μm. Molecular sieve chromatography was performed on a Superdex S20016 / 60 column (GE-Healthcare, Uppsala, Sweden) equilibrated with PBS and injected with 2 ml of the sample from t...
Embodiment 2
[0203] Example 2: Cloning of Der p 1 and Der p 2 allergens
[0204] Complementary DNA (cDNA) encoding the allergens Der p 1 and Der p 2, in each case, were cloned by reverse transcription using mRNA isolated from dust mite as template and specific primers, followed by amplification using PCR . mRNA was isolated from 100 mg of D. dust mite body (Laboratiorios Leti, Madrid, Spain) using the Quick Prep MicroRNA Purification Kit (GE-Healthcare). cDNA was obtained by reverse transcription of mRNA using First Strand cDNA Synthesis Kit (GE-Healthcare).
[0205] Primers consist of a hybridizing region, various cleavage sites (underlined below) for different restriction enzymes, and anchor nucleotides. The PCR amplification reaction had the following components in a reaction volume of 50 μl: Amplification buffer x 10, 5 μl; 200 μm dNTPs; 100 μm of various oligonucleotide primers; 2.5 units Taq polymerase (Pfx DNA polymerase, Invitrogen); 1 ng DNA template and make up to 50 μl of s...
Embodiment 3
[0212] Example 3: Expression and Purification of Recombinant Der p 2
[0213] Transformed with the corresponding plasmid by the Hanahan method [(21) Hanahan, D. (1983). Studies on transformation of Escherichia coli with plasmids (research on transforming E. coli with plasmids). Escherichia coli BL21(DE3) cells were plated on Petri plates containing LB medium supplemented with 200 μg / ml ampicillin. 50 ml of the same medium were pre-inoculated from cell colonies and incubated overnight at 37°C with agitation (260 rpm). Inoculate 1 liter of the same medium with the pre-inoculum, starting from an optical density (600 nm) of 0.2. It was incubated at 37°C with agitation until an optical density (600 nm) of 0.6 was reached (approximately 90 minutes), at which point induction was performed using isopropyl-thio-β-galactoside (IPTG) at a final concentration of 0.6 mM . After a 3 hour induction period, cells were collected by centrifugation.
[0214] Cells were centrifuged at 10000...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com