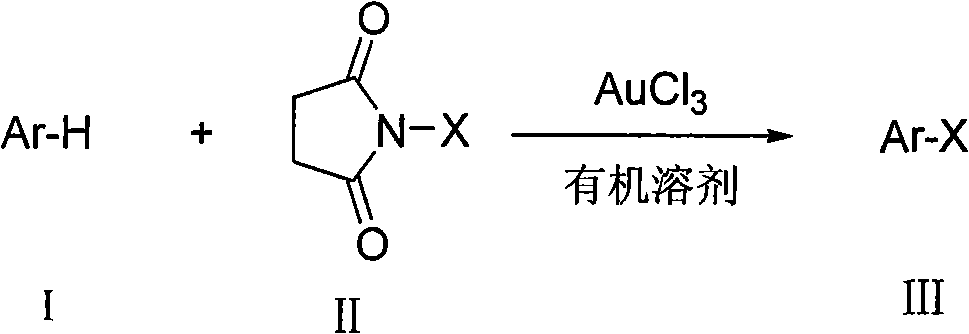
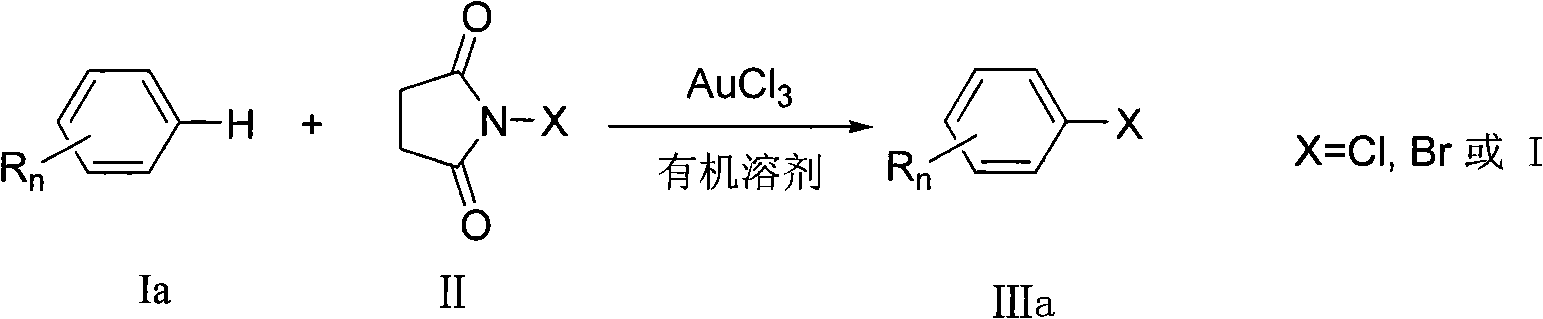
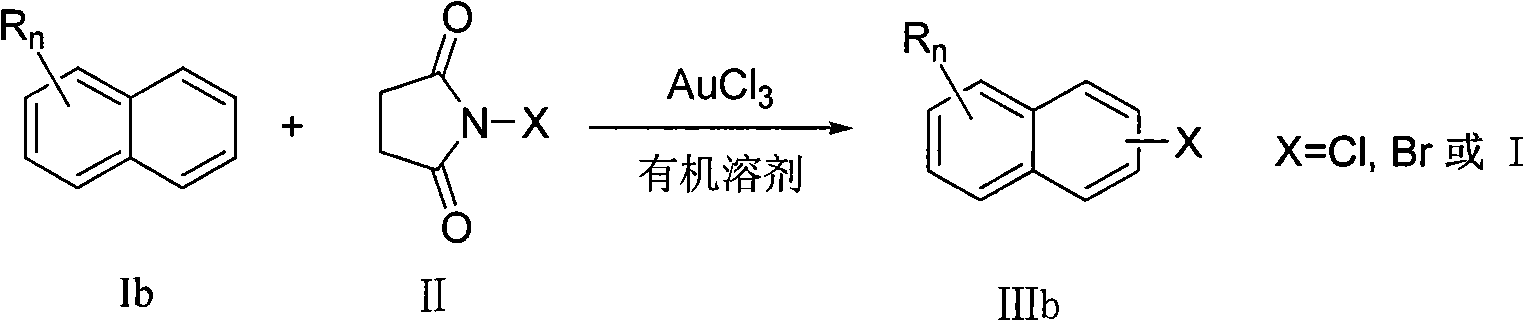
Preparation method of aromatic halogenated compound
A technology of aromatic halogenated compounds, which is applied in the field of preparation of aromatic halogenated compounds, can solve problems such as difficult operation and severe reaction conditions, and achieve the effects of high reaction efficiency, low reaction cost, and convenient and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of p-Bromoanisole
[0033] Add 534mg (i.e. 3mmol) N-bromosuccinimide in the long tube type reaction bottle of 25mL, add 6mL1,2-dichloroethane solvent, then take by weighing 324mg (i.e. 3mmol) anisole, finally Add 0.1 mg of gold trichloride (dissolve 1 mg of gold trichloride in 1 mL of 1,2-dichloroethane, take out 0.1 mL with a micro syringe and inject into the system), heat the reaction at 80°C, and wait for the completion of the reaction as monitored by GC-MS (about 20 hours). Concentrate after the reaction, and use petroleum ether as an eluent for column chromatography purification to obtain p-bromoanisole, whose structure is shown in the following formula:
[0034]
[0035] The compound is a colorless liquid with a yield of 96%, and its NMR data are as follows:
[0036] 1 H NMR (300MHz, CDCl 3 )δ7.37(d, 2H, J=8.8Hz), 6.78(d, 2H, J=8.8Hz), 3.78(s, 3H); 13 C NMR (50MHz, CDCl 3 )δ158.6, 132.1, 115.6, 112.7, 55.3;
Embodiment 2
[0038] Synthesis of methyl 2-methoxy-5-bromobenzoate
[0039] Add 534mg (3mmol) N-bromosuccinimide, 1mg (0.003mmol) of gold trichloride to a 25mL long tube reaction flask, add 6mL 1,2-dichloroethane solvent, and finally weigh 498 mg (3 mmol) of methyl 2-methoxybenzoate was reacted at 80° C., and the reaction was completed by GC-MS monitoring (about 23 hours). Concentrate after the reaction, and use sherwood oil: ethyl acetate volume ratio as the eluent column chromatography purification of 20: 1, can obtain 2-methoxy-5-bromobenzoic acid methyl ester, its structure is shown in the following formula:
[0040]
[0041] The compound is a colorless liquid with a yield of 96%, and its NMR data are as follows:
[0042] 1 H NMR (200MHz, CDCl 3 )δ7.90(d, 1H, J=2.6Hz), 7.55(dd, 1H, J 1 =2.6Hz,J 2 =8.9Hz), 6.87(d, 1H, J=8.9Hz), 3.89(s, 6H); 13 C NMR (50MHz, CDCl 3 )δ165.1, 158.1, 135.9, 134.1, 121.6, 113.8, 112.1, 56.1, 52.1.
Embodiment 3
[0044] Synthesis of 4-bromo-1,2-methylenedioxybenzene
[0045] Add 356mg (2mmol) N-bromosuccinimide, 3mg (0.01mmol) of gold trichloride to a 25mL long-tube reaction flask, add 4mL of 1,2-dichloroethane solvent, and finally weigh 244 mg (2 mmol) of 1,2-methylenedioxybenzene was reacted at room temperature, and the reaction was completed by GC-MS monitoring (about 4 hours). Concentrate after the reaction, and purify by column chromatography using petroleum ether as the eluent to obtain 4-bromo-1,2-methylenedioxybenzene, whose structure is shown in the following formula:
[0046]
[0047] The compound is a colorless liquid with a yield of 92%, and its NMR data are as follows:
[0048] 1 H NMR (200MHz, CDCl 3 )δ6.98~6.92(m, 2H), 6.69(d, 1H, J=8.7Hz), 5.97(s, 2H); 13 CNMR (50MHz, CDCl 3 )δ146.9, 124.3, 113.0, 112.2, 110.3, 109.5, 101.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com