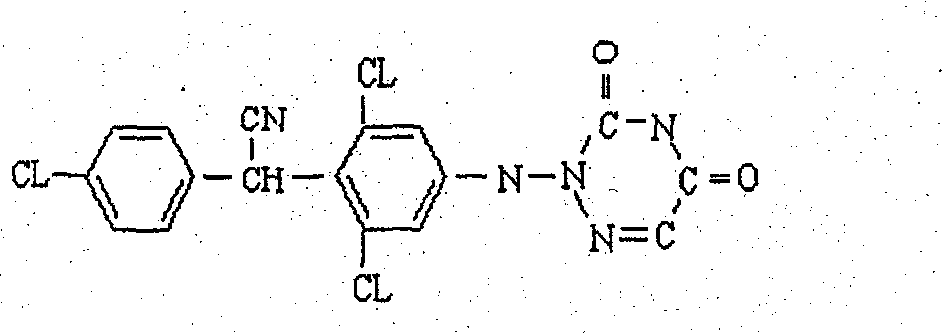
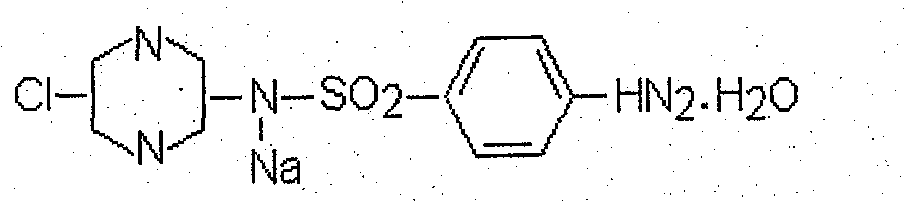
Anticoccidiosis nano suspension and preparation method thereof
A nano-suspension, anti-coccidial technology, applied in the directions of medical preparations, pharmaceutical formulations, anti-infectives, etc. containing active ingredients, to achieve the effects of improving bioavailability, prolonging residence time, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The prescription of diclazuril, maduramycin ammonium, sulfachlorpyrazine sodium nanosuspension:
[0037] components
Dosage
Diclazuril
0.5% (W / V)
Ammonium Maduramycin
2.0% (W / V)
Sodium sulfachlorpyrazine
5.0% (W / V)
Polysorbate-80
1.0% (V / V)
polyethylene glycol-400
0.5% (V / V)
Sodium carboxymethyl cellulose
0.5% (W / V)
0.1% (W / V)
sodium bicitrate
0.05% (W / V)
5% (V / V)
30% (V / V)
Add to 100.0%
[0038] The preparation method of diclazuril, maduramycin ammonium, sulfachlorpyrazine sodium composite nanosuspension:
[0039] (1) First dissolve sodium bicitrate and anhydrous sodium sulfite into a small amount of purified water, stir and dissolve to obtain solution A.
[0040] (2) Diclazuril was added into dimethylacetamide to fully dissolve, then ammonium maduramycin w...
Embodiment 2
[0047] The prescription of diclazuril, maduramycin ammonium, sulfachlorpyrazine sodium nanosuspension:
[0048] components
Dosage
Diclazuril
0.5% (W / V)
Ammonium Maduramycin
2.0% (W / V)
Sodium sulfachlorpyrazine
5.0% (W / V)
Glycerol formal
10.0% (V / V)
Sodium carboxymethyl cellulose
0.5% (W / V)
0.1% (W / V)
sodium bicitrate
2.0% (W / V)
5% (V / V)
30% (V / V)
Add to 100.0%
[0049] The preparation method of diclazuril, maduramycin ammonium, sulfachlorpyrazine sodium composite nanosuspension:
[0050] (1) First dissolve sodium bicitrate and anhydrous sodium sulfite into a small amount of purified water, stir and dissolve to obtain solution A.
[0051] (2) Diclazuril was added into dimethylacetamide to fully dissolve, then ammonium maduramycin was added to completely dissolve and stirred ev...
Embodiment 3
[0057] Example 3, clinical efficacy test of diclazuril, maduramycin ammonium, and sulfachlorpyrazine sodium composite nanosuspension
[0058] In order to verify the efficacy of the compound nanosuspension of diclazuril, maduramycin ammonium and sulfachlorpyrazine sodium, a laboratory efficacy verification test was carried out. Using AA broiler chickens, when they were raised to 11 days old, they were inoculated with 100,000 sporulated oocysts of Eimeria tenella by artificial crop infection, and the anti-coccidia effect of the compound nanosuspension was determined, and Group A: diclazuril solution (5mg / ml); group B: diclazuril, maduramycin ammonium solution (containing diclazuril diclazuril: 5mg / ml; maduramycin ammonium: 30mg / ml); C group: 10% sulfachlorpyrazine sodium soluble powder is used as contrast drug. The test results are as follows:
[0059] Table 1: Experimental results of diclazuril, maduramycin ammonium, and sulfachlorpyrazine sodium nanosuspensions
[0060] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com