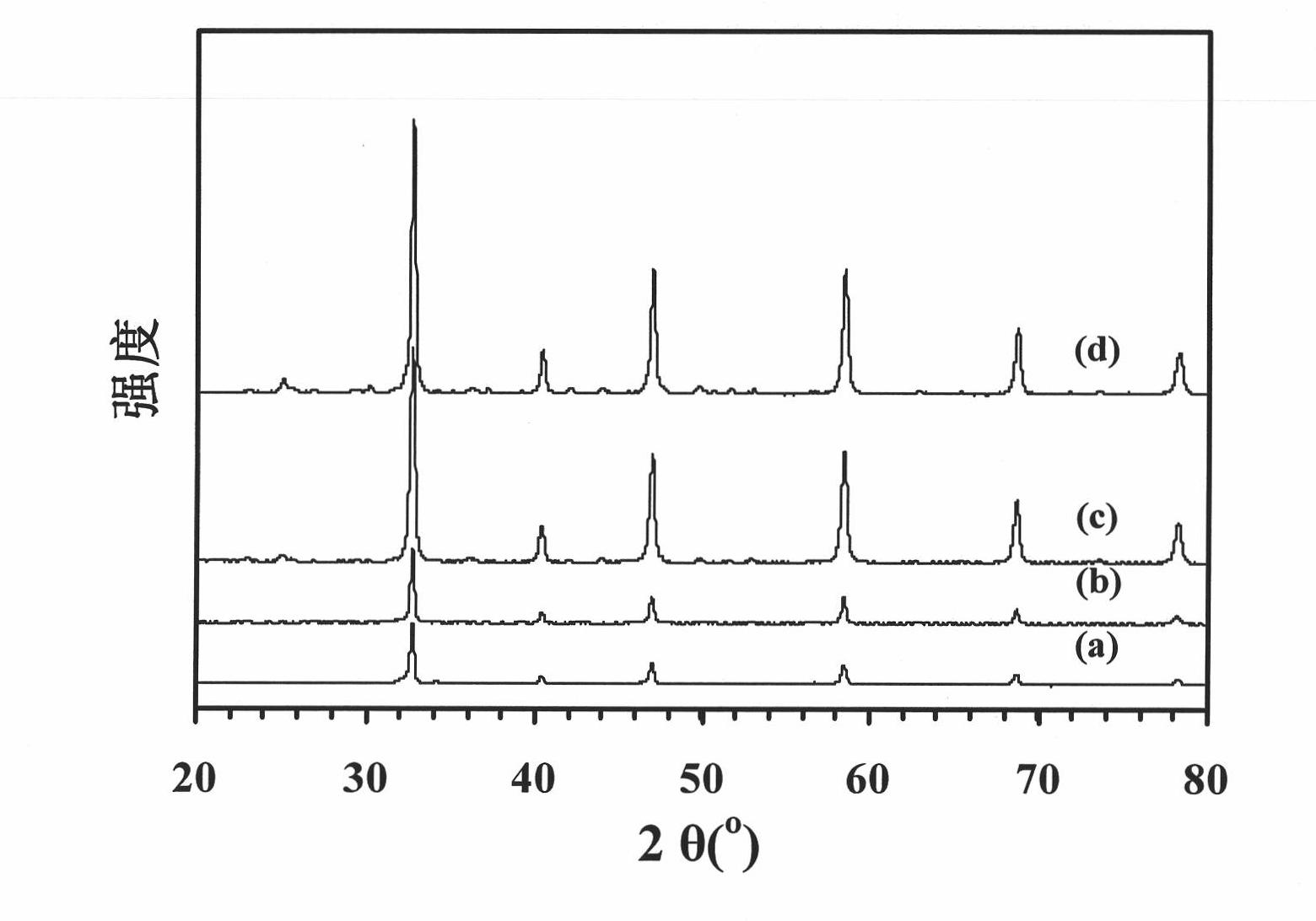
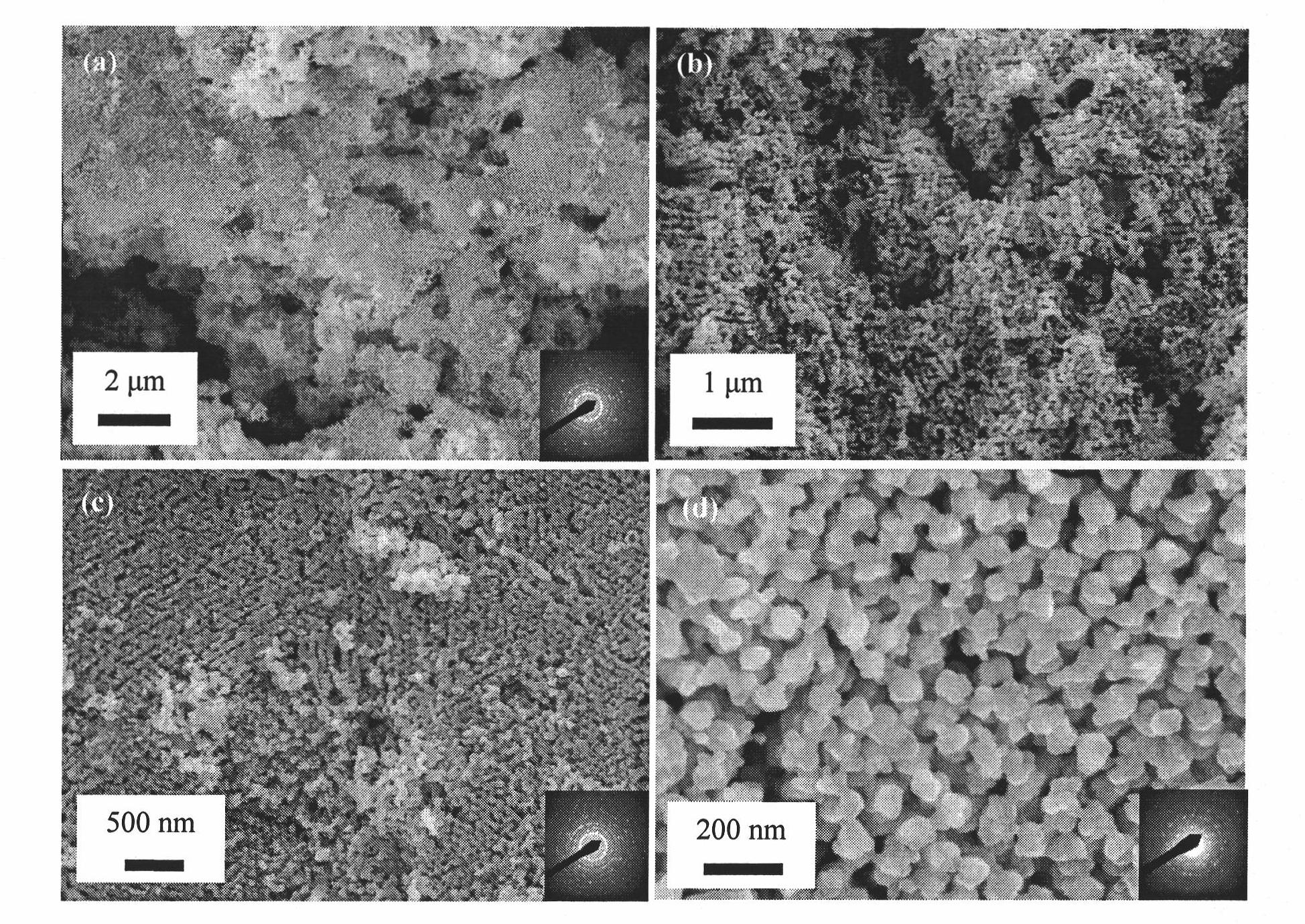
New method for preparing polycrystalline SrFeO3 with three-dimensional ordered macroporous structure
A technology with three-dimensional order and pore structure, applied in chemical instruments and methods, inorganic chemistry, iron compounds, etc., to achieve the effects of good thermal stability of products, huge application prospects, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: Under stirring conditions, 0.015mol Sr(NO 3 ) 2 and 0.015mol Fe(NO 3 ) 3 9H 2 Dissolve O in 11mL deionized water, first add 3.6g citric acid, then add 0.72g lysine after it is completely dissolved, then add 1mL methanol, set the volume of the solution to 20mL, and keep stirring until a uniform metal ion complex is formed Solution; Weigh 2g PMMA microspheres as a hard template, slowly pour into the beaker containing the above mixed solution and soak for 12h, then suction filter, and dry the obtained sample at room temperature for more than 12h; finally put the sample into a magnetic boat and place it in a tube type furnace, first in N 2 Under the atmosphere, raise from room temperature to 600°C at a rate of 1°C / min and keep the temperature constant for 2 hours. After the temperature drops below 70°C, switch to air atmosphere, then raise it to 300°C at a rate of 1°C / min and keep the temperature constant for 1 hour, then Continue to raise the temperature to...
Embodiment 2
[0014] Example 2: Under stirring conditions, 0.015mol Sr(NO 3 )2 and 0.015mol Fe(NO 3 ) 3 9H 2 O was dissolved in 11mL of deionized water, and 3mL of ethylene glycol was added first, and then 3.6g of citric acid was added successively after the miscibility was complete, and the stirring was continued until a uniform metal ion complex solution was formed. At this time, the solution volume was 21mL; Methyl acrylate (PMMA) microspheres were used as a hard template, slowly poured into the beaker containing the above mixed solution and soaked for 12 hours, then suction filtered, and the obtained sample was dried at room temperature for more than 12 hours; finally, the sample was placed in a magnetic boat tube furnace, first in N 2 Under the atmosphere, raise from room temperature to 600°C at a rate of 1°C / min and keep the temperature constant for 2 hours. After the temperature drops below 70°C, switch to air atmosphere, then raise it to 300°C at a rate of 1°C / min and keep the te...
Embodiment 3
[0015] Embodiment 3: under stirring condition, 0.015mol Sr(NO 3 ) 2 and 0.015mol Fe(NO 3 ) 3 9H 2 O was dissolved in 10mL of deionized water, firstly added 3mL of ethylene glycol, and then added 3.6g of citric acid and 0.72g of sucrose successively after the miscibility was complete, and continued to stir until a uniform metal ion complex solution was formed. At this time, the volume of the solution was 20mL; Take 2g of PMMA microspheres as a hard template, slowly pour them into a beaker containing the above mixed solution and soak for 12 hours, then filter with suction, and dry the obtained sample at room temperature for more than 12 hours; finally put the sample into a magnetic boat and place it in a tube furnace , first at N 2 Under the atmosphere, raise from room temperature to 600°C at a rate of 1°C / min and keep the temperature constant for 2 hours. After the temperature drops below 70°C, switch to air atmosphere, then raise it to 300°C at a rate of 1°C / min and keep t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com