Salicylamide ester type derivative and preparation method and application thereof
A technology of salicylamide and derivatives, which is applied in the field of salicylamide ester derivatives, and can solve problems such as researches that have not been reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
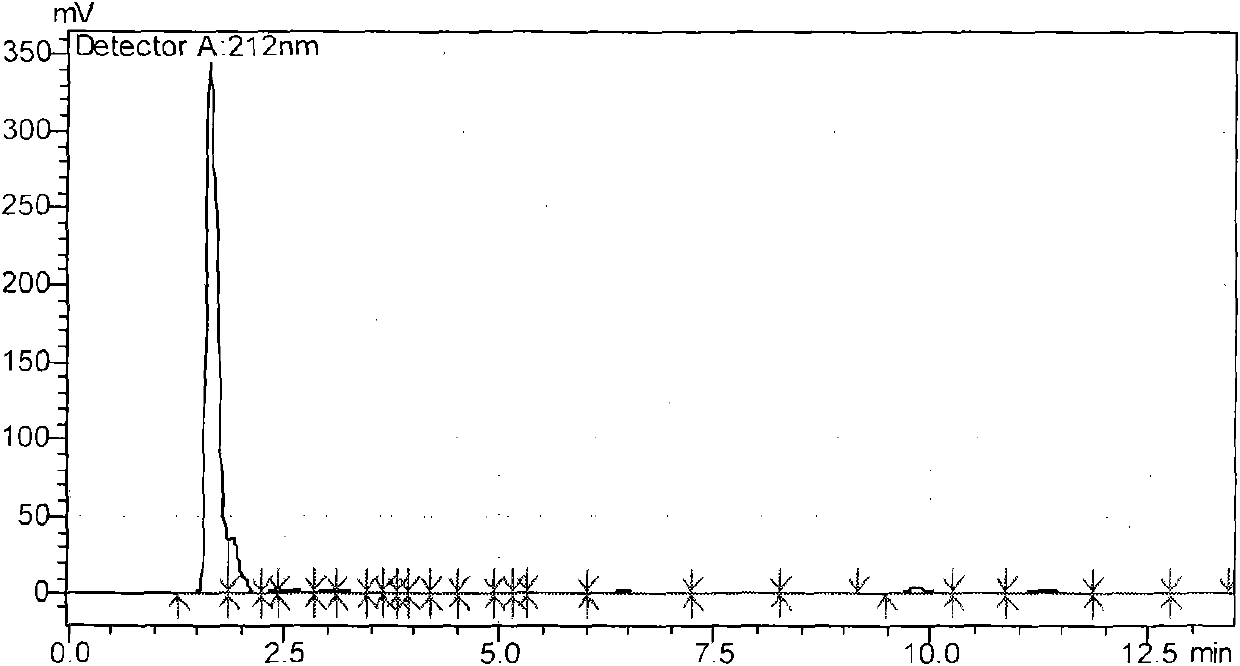
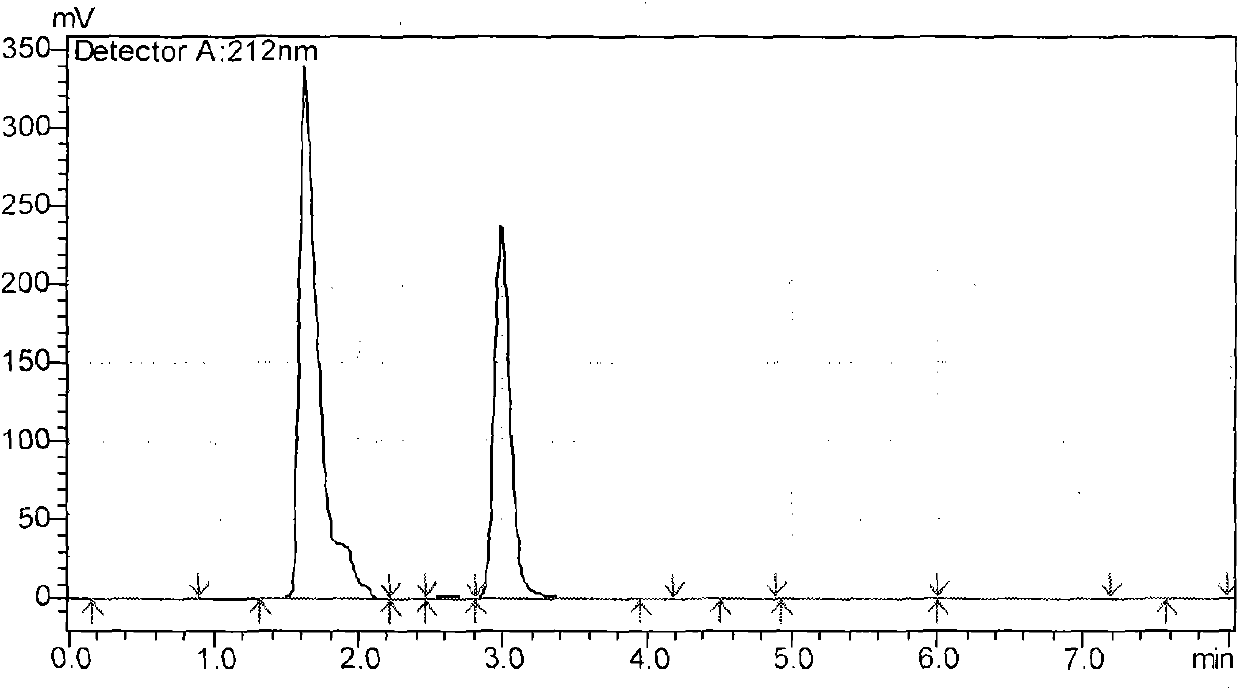
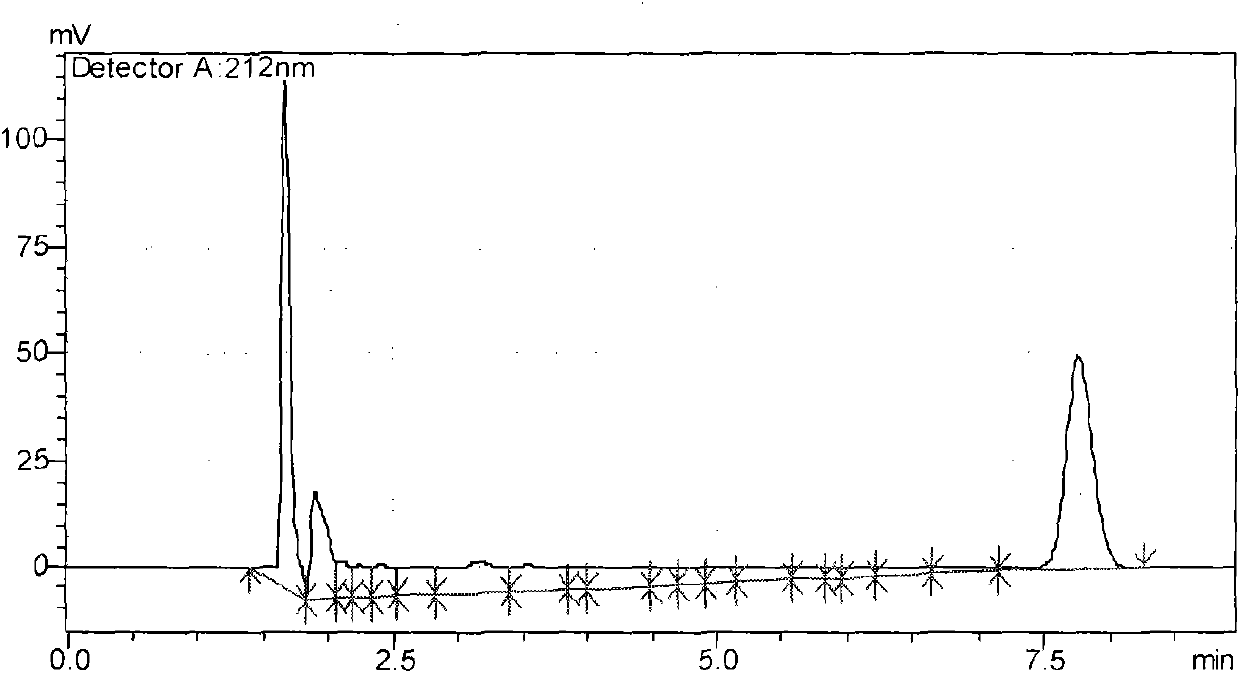
Image
Examples
Embodiment 1
[0021] The synthesis of embodiment 1 niclosamide valproate
[0022] 1.1 Preparation of valproyl chloride
[0023] Reaction formula
[0024]
[0025] In reaction flask, add valproic acid (5.0g, 20.8mmol), SOCl 2 (40mL), add 2 drops of DMF as a catalyst, stir the reaction at 85°C for 4h, then distill off excess SOCl under reduced pressure 2 , and added a small amount of benzene and distilled twice to remove residual SOCl 2 , to obtain valproyl chloride for subsequent use.
[0026] 1.2 Synthesis of niclosamide valproate
[0027] Reaction formula
[0028]
[0029] reaction steps
[0030] Add niclosamide (7.0 g, 21.0 mmol), acetone (60 mL), and triethylamine (4 mL) into the reaction flask, and heat to dissolve under stirring. Then the valproyl chloride (3.4g, 0.021mol) prepared in 1.1 was dissolved in 15mL of dry acetone, and was added dropwise to the niclosamide acetone solution with a constant pressure dropping funnel. TLC detection [V (petroleum ether): V (ethyl ac...
Embodiment 2
[0031] The synthesis of embodiment 2N-(3-chlorophenyl)-2-hydroxyl-4-methoxybenzamide valproate
[0032] 2.1 Synthesis of N-(3-chlorophenyl)-2-hydroxyl-4-methoxybenzamide
[0033] Reaction formula
[0034]
[0035] reaction steps
[0036] Add 4-methoxysalicylic acid (6.0g, 35.7mmol), m-chloroaniline (5.0g, 39.2mmol) and solvent chlorobenzene (80mL) in 250mL three-necked bottle, install reflux condenser, heat and stir until After complete dissolution, the temperature was raised to 135°C to maintain reflux. Phosphorus trichloride (2.4g, 17.5mmol) dissolved in chlorobenzene was slowly added dropwise, and kept at 135°C for a constant temperature reaction for 3h, and detected by TLC [V (petroleum ether): V (ethyl acetate) = 3:1 as developing solvent] Shows complete reaction. Suction filtration while hot, cool and precipitate solid, filter to obtain white solid, wash 2-3 times with 50ml, drain and recrystallize with acetone, the final white crystal, after drying, obtain N-(3-c...
Embodiment 3
[0042] The synthesis of embodiment 3N-(3-bromophenyl)-2-hydroxyl-4-methoxybenzamide valproate
[0043] 3.1 Synthesis of N-(3-bromophenyl)-2-hydroxy-4-methoxybenzamide
[0044] Reaction formula
[0045]
[0046] reaction steps
[0047] According to the method of 2.1 in Example 2, white crystals of N-(3-bromophenyl)-2-hydroxy-4-methoxybenzamide were obtained with a yield of 64.0%, m.p.163.0-163.8°C. 1 H NMR (DMSO, 300MHz) δ: 12.18 (s, 1H, OH), 10.29 (s, 1H, NH), 8.03 (s, 1H, ArH), 7.97 (d, J=9.0Hz, 1H, ArH), 7.68~7.64(m, 1H, ArH), 7.35(d, J=7.8Hz, 2H, ArH), 6.59(dd, J=9.0Hz, J=2.4Hz, 1H, ArH), 6.51(d, J= 2.4Hz, 1H, ArH), 3.80(s, 3H, OCH 3 ); 13 C-NMR (DMSO, 75MHz) δ: 167.7, 164.4, 162.0, 140.3, 131.0, 130.7, 127.0, 123.8, 121.9, 120.2, 109.5, 106.9, 101.8, 55.9; IR (KBr, cm -1 )υ: 3319.7, 3073.6, 2363.6, 1631.4, 1475.3, 1435.7, 1320.5, 1202.7, 1131.0, 1096.4, 1031.3, 994.0, 961.8, 858.8, 830.6, 774.1; ESI-Mass for C 14 h 12 BrNO 3 : m / z(M + +H)322.16.
[0048] 3.2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com