Theasapogenol derivative with antibacterial effect as well as preparation method and application thereof
A technology of tea saponin and derivatives, which is applied in the field of health and medicine, can solve the problems of unfavorable entry into bacteria and fungal cells, lack of direct sterilization, large molecular weight structure, etc., and achieve small structure, improved antibacterial activity, and simple preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
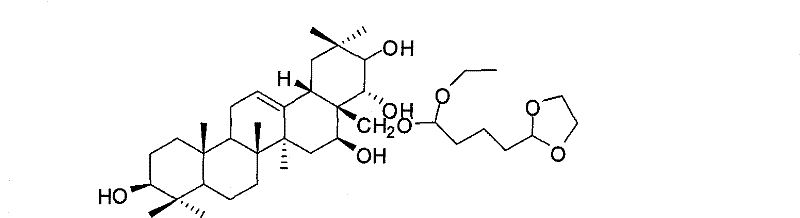
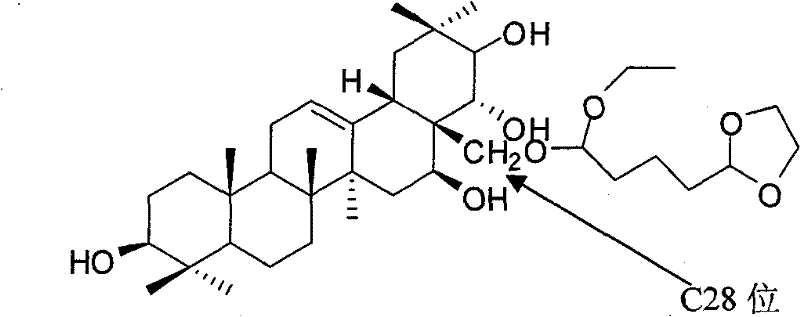
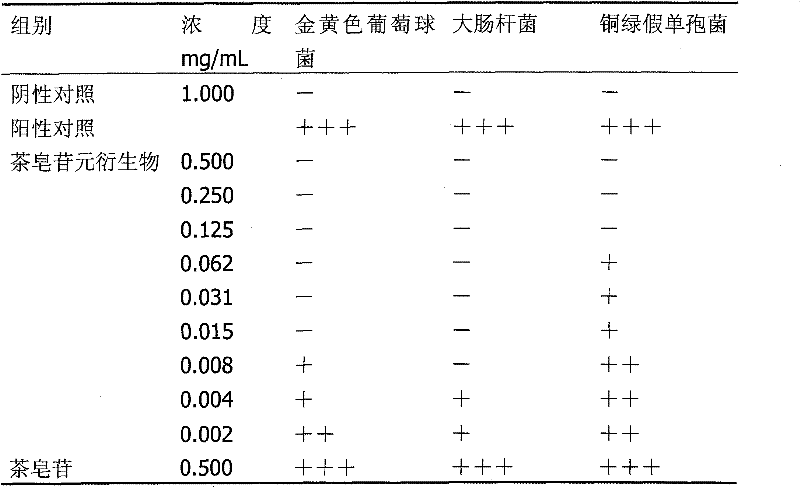
[0032] Theosapogenin (C 30 h 50 o 5 ) solid 100g, added to 300mL of pyridine, heated to 50°C to dissolve, added triphenylchloromethane (56.7g) in an equimolar amount to the tea saponin, kept warm (50°C) and stirred for 8 hours; added tea saponin Acetic anhydride (41.6g) with 2 times the molar amount was stirred at 50°C for 15h; 9.4mL of formic acid was added, the reaction temperature was 100°C, and refluxed for 1h; then 4-dioxolane-1,1-diethyloxybutyl was added 45g of alkane, reaction temperature 70°C, stirred for 9h; cooled to 20°C, added 56g of potassium carbonate, stirred for 1h, evaporated pyridine under reduced pressure, added 1000mL of water to wash, added methanol to crystallize water-insoluble matter, dried in vacuo, prepared 34 g of thea-sapogenin derivatives were obtained.
[0033] The structural analysis of the product shows that MS: m / z 662; the elemental composition is: C70.66%, H10.03%, O19.31%, and its molecular formula is C 39 h 66 o 8 , 1 H-NMR with 13...
Embodiment 2
[0035] Theosapogenin (C 30 h 50 o 5 ) solid 100g, added to 300mL of pyridine, dissolved at room temperature (25°C), added triphenylchloromethane (85g) according to 1.5 times the molar amount of teasapogenin, and kept stirring for 12 hours; Amount of acetic anhydride (83.2g), stirred at 70°C for 10h; added 14mL of formic acid, the reaction temperature was 105°C, and refluxed for 1h; then added 45g of 4-dioxolane-1,1-diethyloxybutane, the reaction temperature Stir and react at 75°C for 6h; after cooling to 30°C, add 112g of potassium carbonate, stir for 1h, evaporate pyridine under reduced pressure, add 1000mL of water to wash, add water-insoluble matter into methanol to crystallize, and dry in vacuo to obtain 49g of tea saponin meta derivatives.
[0036] The structural analysis data of the product are the same as in Example 1. The product proved to be 28-(4-dioxolane-1-ethyloxybutoxy)-teasapogenol.
Embodiment 3
[0038] Theosapogenin (C 30 h 50 o 5 ) solid 100g, added to 300mL of pyridine, heated to 40°C to dissolve, added triphenylchloromethane (68g) according to 1.2 times the molar amount of teasapogenin, and kept stirring for 16 hours; Acetic anhydride (62.4g) was stirred at 80°C for 8h; 11.3mL of formic acid was added, the reaction temperature was 110°C, and refluxed for 1h; Stir at 80°C for 3 hours; after cooling to 25°C, add 84g of potassium carbonate, stir for 1h, evaporate pyridine under reduced pressure, add 1000mL of water to wash, add methanol to crystallize water-insoluble matter, and vacuum dry to obtain 43g of tea saponin meta derivatives.
[0039] The structural analysis data of the product are the same as in Example 1. The product proved to be 28-(4-dioxolane-1-ethyloxybutoxy)-teasapogenol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com