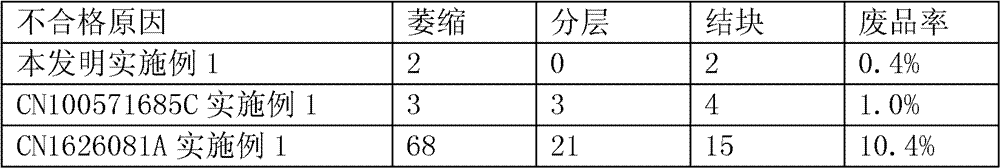
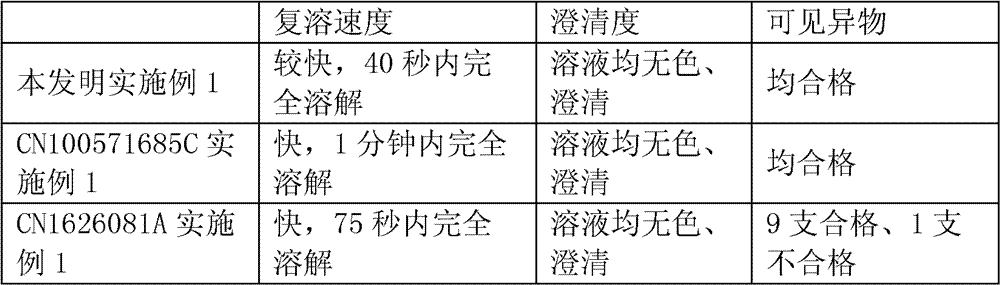
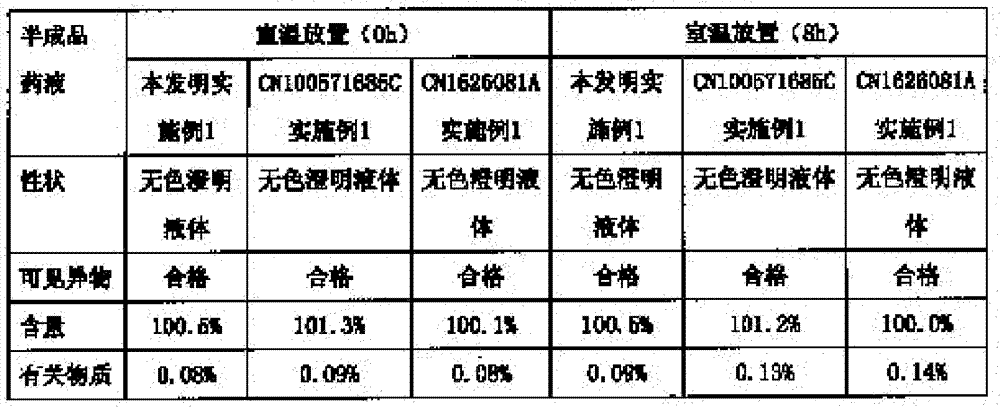
Ropivacaine mesylate freeze-dried powder injection
A technology for ropivacaine mesylate and freeze-dried powder injection, which is applied in the field of western medicine preparations, can solve the problems of unsuitability for large-scale industrial production, high cost input, poor product appearance, etc., and achieves loose and uniform appearance, fast cooling rate, The effect of avoiding security risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Preparation of ropivacaine mesylate freeze-dried powder injection
[0024] Prescription: Ropivacaine Mesylate 89.4g
[0025] Add water for injection to 1000mL
[0026] 1000 made
[0027] Preparation: Weigh 89.4g of ropivacaine mesylate, add 80% of the prescribed amount of water for injection cooled to room temperature, stir to dissolve completely, adjust the pH of the liquid to 4-7 with sodium hydroxide or phosphoric acid, add 0.5‰ of injection Use activated carbon, stir and adsorb for 10 minutes, then decarbonize and filter, add water for injection to 1000mL, and stir evenly. Afterwards, it is filtered through a 0.22 μm sterile filter membrane in two stages of sterilization, and the filtrate is sampled to detect the content of the semi-finished product. According to the content, the specified amount of liquid medicine is filled into clean vials, half-filled with rubber stoppers, and placed in a freeze dryer for freeze-drying. The freeze-drying process is a...
Embodiment 2
[0032] Example 2 Preparation of ropivacaine mesylate freeze-dried powder injection
[0033] Prescription: Ropivacaine Mesylate 47.7g
[0034] Add water for injection to 1000mL
[0035] 1000 made
[0036] Preparation: Weigh 47.7g of ropivacaine mesylate, add 80% of the prescribed amount of water for injection cooled to room temperature, stir to dissolve completely, adjust the pH of the liquid to 4-7 with sodium hydroxide or phosphoric acid, add 1‰ of the injection Use activated carbon, stir and adsorb for 10 minutes, then decarbonize and filter, add water for injection to 1000mL, and stir evenly. Afterwards, it is filtered through a 0.22 μm sterile filter membrane in two stages of sterilization, and the filtrate is sampled to detect the content of the semi-finished product. According to the content, the specified amount of liquid medicine is filled into clean vials, half-filled with rubber stoppers, and placed in a freeze dryer for freeze-drying. The freeze-drying process is...
Embodiment 3
[0041]Example 3 Preparation of ropivacaine mesylate freeze-dried powder injection
[0042] Prescription: Ropivacaine Mesylate 89.4g
[0043] Add water for injection to 500mL
[0044] 1000 made
[0045] Preparation: Weigh 89.4g of ropivacaine mesylate, add 80% of the prescribed amount of water for injection cooled to room temperature, stir to dissolve completely, adjust the pH of the liquid to 4-7 with sodium hydroxide or phosphoric acid, add 1‰ of the injection Use activated carbon, stir and adsorb for 10 minutes, then decarbonize and filter, add water for injection to 500mL, and stir evenly. Afterwards, it is filtered through a 0.22 μm sterile filter membrane in two stages of sterilization, and the filtrate is sampled to detect the content of the semi-finished product. According to the content, the specified amount of liquid medicine is filled into clean vials, half-filled with rubber stoppers, and placed in a freeze dryer for freeze-drying. The freeze-drying process is as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com