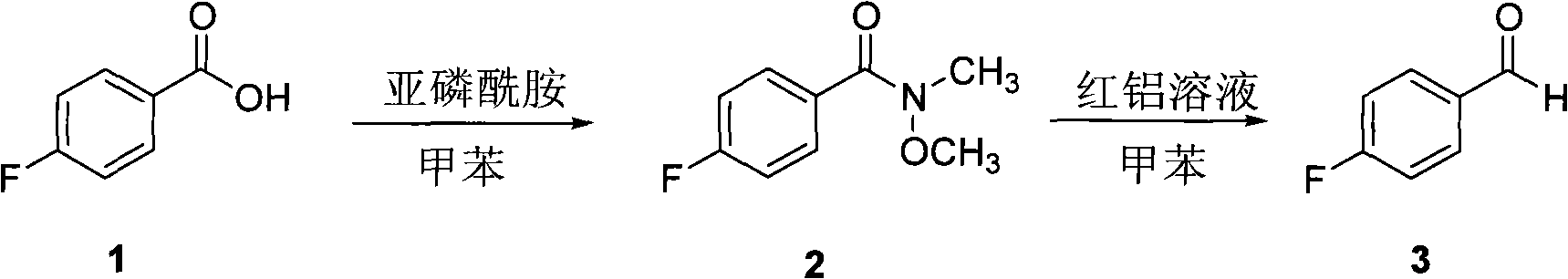
Method for preparing p-fluorobenzaldehyde
A technology of p-fluorobenzaldehyde and p-fluorobenzoic acid, which is applied in the field of preparation of pharmaceutical intermediate p-fluorobenzaldehyde, can solve the problems of separation of ortho isomers, high toxicity of solvents, etc., and achieve few synthesis steps, single product, Avoid the effects of catalyst use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
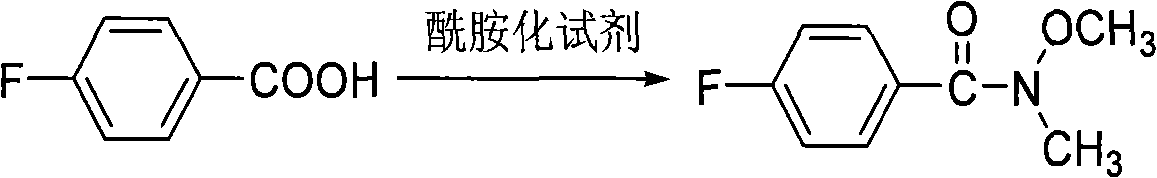
[0029] (1) Preparation of N-methyl-N-methoxy p-fluorobenzamide
[0030] Under nitrogen protection, add toluene (15mL), p-fluorobenzoic acid (6.05g, 0.043mol), phosphoramidite P(NMeOMe) in sequence in a 100mL two-necked flask 3 (3.76g, 0.018mol), the mixture was reacted at 60°C for 30 minutes. After the completion of the reaction detected by TLC, the reaction was quenched with saturated sodium bicarbonate solution (100mL); extracted with ether (15mL×3), the organic phase was washed with water (10mL×2) and saturated brine (20mL) successively, and dried over anhydrous sodium sulfate ; The solvent was evaporated under reduced pressure and separated by column chromatography (petroleum ether / ethyl acetate=4:1) to obtain a colorless liquid (4.72g), which was N-methyl-N-methoxy-p-fluorobenzamide. The yield is 90%.
[0031] The N-methyl-N-methoxy p-fluorobenzamide of above-mentioned synthesis, through 1 HNMR, 13 CNMR, IR detection, the product is a pure target compound. Its variou...
Embodiment 2
[0038] (1) Preparation of N-methyl-N-methoxy p-fluorobenzamide
[0039] Add p-fluorobenzoic acid (6.05g, 0.043mol) and PhP 3 (11.27g, 0.043mol), then THF (15mL) was added, the mixture was cooled to about 0°C, NBS (8.42g, 0.047mol) was added in batches under stirring, and stirred for 30 minutes. Continue to add NH(OMe)Me (4.46g, 0.073mol) to the above reaction solution, after the completion of the reaction was detected by TLC, the reaction was quenched with saturated sodium bicarbonate solution (100mL); ether extraction (15mL×3), organic phase Wash with water (10mL×2) and saturated brine (20mL) once, and dry over anhydrous sodium sulfate; evaporate the solvent under reduced pressure, and separate by column chromatography (petroleum ether / ethyl acetate=4:1) to obtain a colorless liquid (4.52 g), which is N-methyl-N-methoxy-p-fluorobenzamide. The yield was 86%.
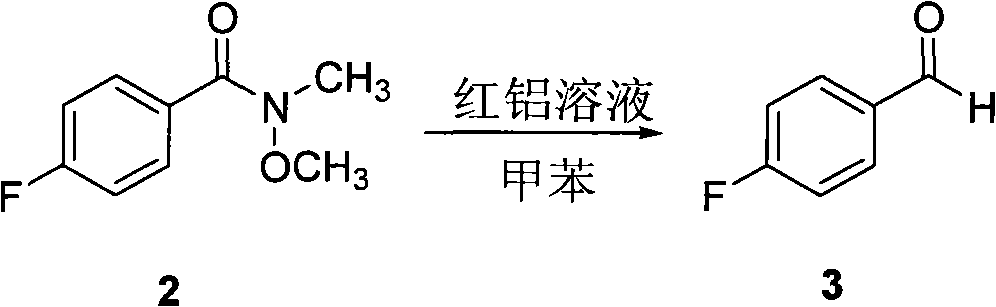
[0040] (2) Preparation of p-fluorobenzaldehyde
[0041] Same as Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com