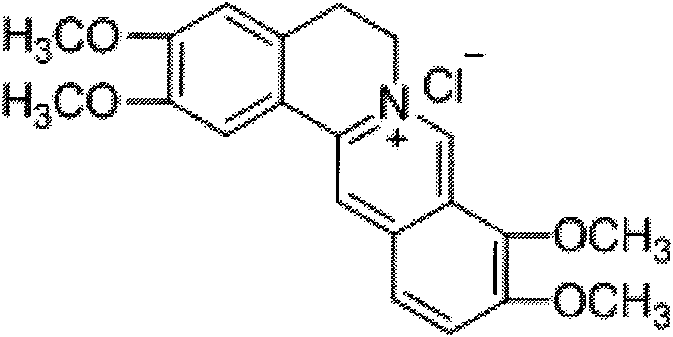
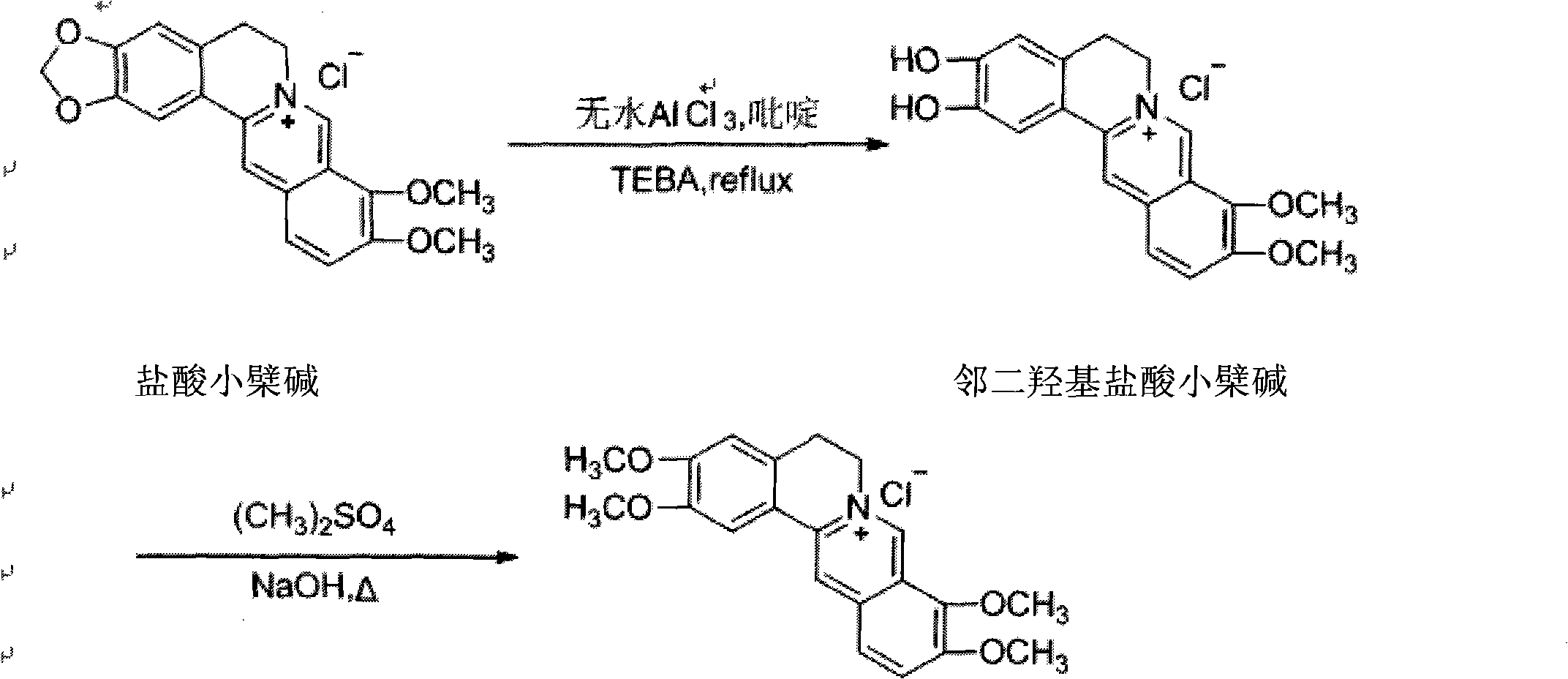
Fibriuretinin synthesis method
A synthetic method, the technology of palmatine, applied in the direction of organic chemistry, etc., can solve the problems of harsh reaction conditions and lengthy synthetic routes, and achieve the effect of simple synthetic conditions, simple synthetic process and few steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1) Preparation of o-dihydroxy berberine hydrochloride:
[0029] In a 250mL three-necked flask equipped with a stirrer and a reflux condenser, 5g of berberine hydrochloride was sequentially added, dissolved in 40mL of methanol, 0.05g of phase transfer catalyst TEBA was added, and 2g of anhydrous aluminum trichloride was added, and dripped under nitrogen protection. Add 2g of pyridine, stir for 10 minutes, and heat to reflux for 4h. The reaction solution was slowly poured into an appropriate amount of ice water, and stirred at the same time, acidified, left to stand, a yellow precipitate was produced, filtered with suction, the filter cake was recrystallized with 70% ethanol and activated carbon, filtered with suction, and dried in a vacuum oven ( The temperature is not more than 60° C.) to obtain 3.5 g of the product o-dihydroxy berberine hydrochloride with a yield of 73.2%.
[0030] Take berberine hydrochloride and dissolve it with an appropriate amount of methanol as ...
Embodiment 2
[0035] 1) Preparation of o-dihydroxy berberine hydrochloride:
[0036] In a 250mL three-necked flask equipped with a stirrer and a reflux condenser, 10g of berberine hydrochloride was added successively, dissolved in 80mL of methanol, 0.1g of phase transfer catalyst TEBA was added, and 4g of anhydrous aluminum trichloride was added. 2.5g of pyridine was added dropwise, and after stirring for 10 minutes, it was heated to reflux for 5h. The reaction solution was slowly poured into an appropriate amount of ice water, and stirred at the same time, acidified, stood still, a yellow precipitate was produced, filtered with suction, the filter cake was recrystallized with 80% ethanol and activated carbon, filtered with suction, and dried in a vacuum oven ( The temperature does not exceed 60° C.) to obtain 6.9 g of the product o-dihydroxy berberine hydrochloride with a yield of 72.9%.
[0037] Take berberine hydrochloride and dissolve it with an appropriate amount of methanol as a refe...
Embodiment 3
[0041] 1) Preparation of o-dihydroxy berberine hydrochloride:
[0042] In a 500mL three-necked flask equipped with a stirrer and a reflux condenser, 15g of berberine hydrochloride was sequentially added, dissolved in 150mL of methanol, 0.2g of a phase transfer catalyst TEBA was added, and 6g of anhydrous aluminum trichloride was added, and dripped under the protection of xenon Add 3g of pyridine, stir for 10 minutes, and heat to reflux for 6h. The reaction solution was slowly poured into an appropriate amount of ice water, and stirred at the same time, acidified, left standing, a yellow precipitate was produced, filtered with suction, the filter cake was recrystallized with 90% ethanol and activated carbon, filtered with suction, and dried in a vacuum oven ( The temperature does not exceed 60° C.) to obtain 9.8 g of the product o-dihydroxy berberine hydrochloride with a yield of 73.1%.
[0043] Take berberine hydrochloride and dissolve it with an appropriate amount of methano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com