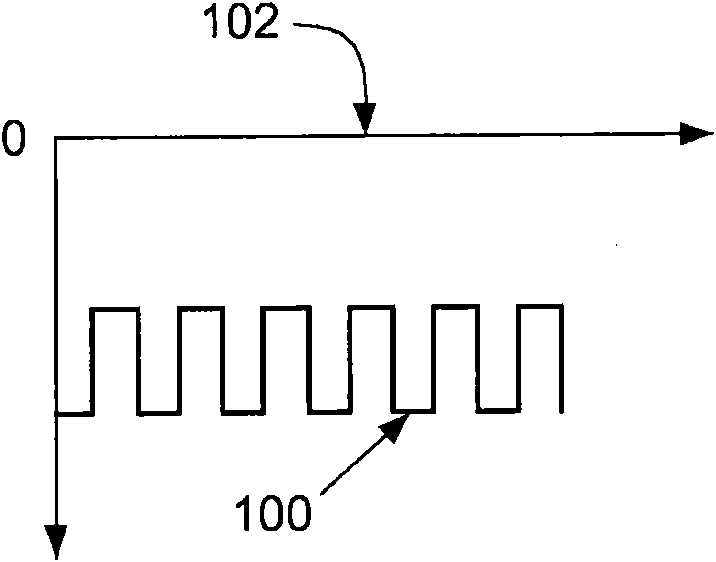
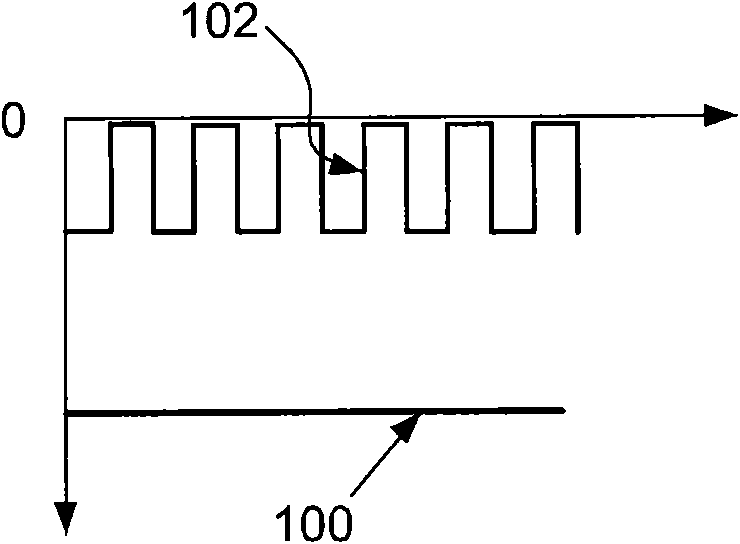
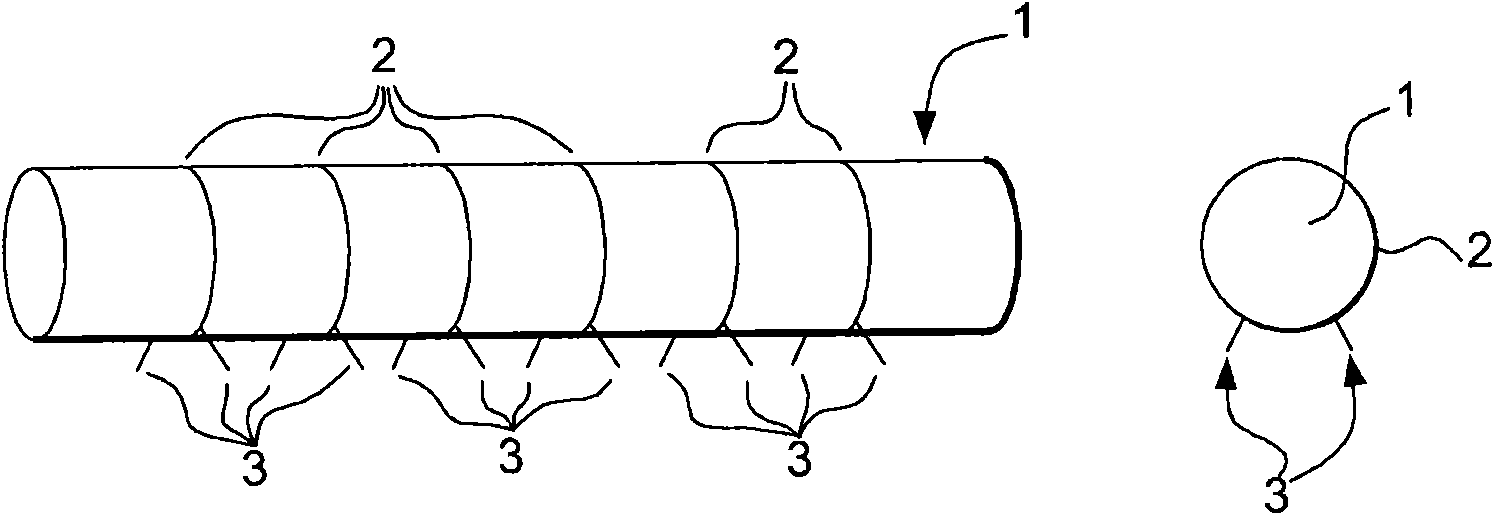
Method for the removal of surface oxides by electron attachment
An oxide, base electrode technology, applied in circuits, furnace types, printed circuits, etc., to solve problems that hinder accurate processing, control surface charges, change electric fields, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] The first experiment was performed using a laboratory scale furnace. The sample used was a flux-free tin-lead solder preform (melting point 183°C) on a grounded copper plate (anode), which was loaded into a furnace and heated at 5% H 2 N 2 heated to 250°C under airflow. When the sample temperature is in equilibrium, a DC voltage is applied between the negative electrode (cathode) and the grounded sample (anode) and gradually increased to about -2kV with a current of 0.3mA. The distance between the two electrodes is about 1 cm. Pressure is ambient atmospheric pressure. It was found that the solder wetted very well indeed on the copper surface. Good wetting of the fluxless solder on the copper surface can never be obtained at such low temperatures without an applied voltage, even in pure H 2 The same is true in , because with pure H 2 The effective temperature for removing tin oxide on tin-based solder is higher than 350°C. Therefore, this result confirms that the ...
Embodiment 2
[0111] Electron attachment assisted hydrogen fluxless soldering of several cathode materials was investigated with the same setup as in Example 1 by using the field emission mechanism. The results of the study are given in Table I.
[0112] As shown in Table I, the best results were obtained using a Ni / Cr cathode: it provided the highest melting efficiency and thus the shortest wetting time. It is believed that the Ni / Cr cathode produces a relatively large amount of electrons and has a suitable electron energy level compared to other cathode materials.
[0113] Table I: 250°C and 20%H 2 Effect of lower cathode material on wetting time
[0114] Cathode rod (1 / 16" diameter) material with tip
[0115] Nichrome
Embodiment 3
[0117] This example was carried out to investigate the efficiency of electron generation by the thermal-field emission method. A graphite rod with a diameter of 3 mm and a machined tip protruding from its surface with a length of several 1 mm was used as the cathode, the geometry of which was similar to Figure 2i shown in . Each protruding machined tip has a tip cone angle of 25 degrees. The graphite rod was heated by resistance using an AC power supply at 5% H 2 and 95%N 2 The gas mixture is heated to about 400 to 500°C. A DC voltage power supply of 5 kV was applied between a graphite cathode with a gap of 1.5 cm therebetween and a copper plate used as an anode. All tips on the graphite rod illuminate, indicating that electrons can be generated uniformly from the tips distributed on the graphite rod. Without heating the graphite rod, no electrons are emitted from the cathode or arc between one of the tips and the anode plate. This suggests that the combination of using...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| current | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com