Method for preparing 2-aminobenzimidazole derivative
A technology for aminobenzimidazole and derivatives, which is applied in the field of preparation of 2-aminobenzimidazole derivatives, can solve the problems of long route, hindering the practical application of the reaction, and difficulty in obtaining reaction raw materials, etc., and achieves the effect of high synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
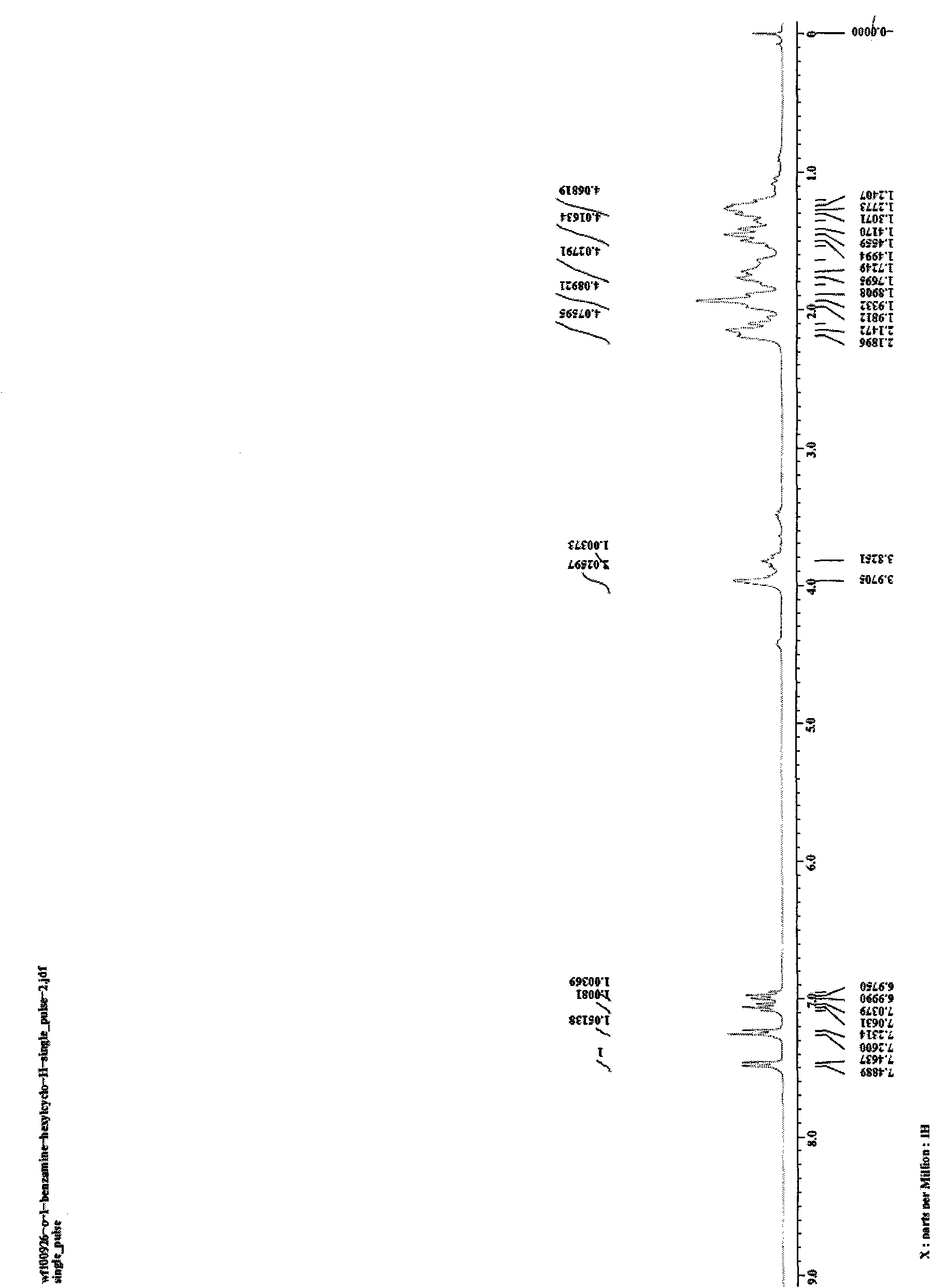
[0023] Embodiment 1, N, the preparation of 1-dicyclohexyl-2-aminobenzimidazole
[0024] To a 20mL reactor, add 2-bromoaniline (0.5mmol, 86mg), cuprous iodide (0.05mmol, 10mg), sodium tert-butoxide (1.0mmol, 96mg), N,N-dicyclohexyl carbon Dicyclohexylcarbodiimide (DCC) (0.5 mmol, 103 mg), and finally 1 mL of NMP was added as a solvent, the reactor was sealed, and reacted at 110° C. for 24 h. After the reaction system was cooled, 2 mL of water and 5 mL of ethyl acetate were added and stirred for 30 min, then extracted three times with 15 mL of ethyl acetate, and the organic phases were combined. The organic phase was washed with saturated brine, and finally dried with anhydrous magnesium sulfate for 2 h. After filtration, the organic phase was rotary evaporated to obtain a crude product. The crude product was separated by column chromatography with petroleum ether: ethyl acetate=3:1 (v / v) as the eluent and silica gel as the adsorption phase to obtain a light brown solid product...
Embodiment 2
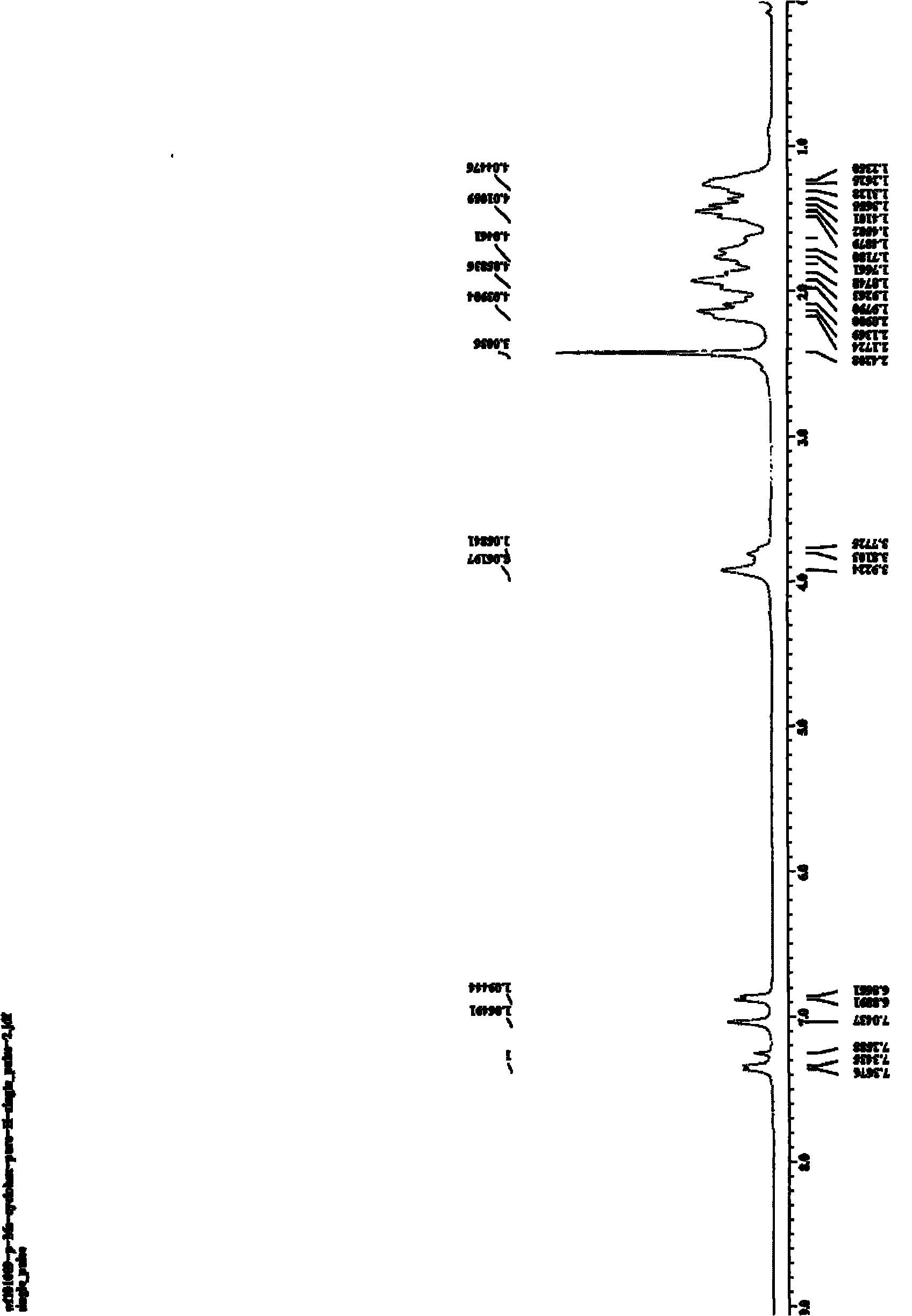
[0027] Embodiment 2, N, the preparation of 1-dicyclohexyl-6-methyl-2-aminobenzimidazole
[0028]Into a 20mL reactor, add 4-methyl-o-bromoaniline (0.50mmol, 62μL), CuI (0.05mmol, 10mg), sodium tert-butoxide (1.0mmol, 96mg), N,N-dicyclohexyl carbon Dicyclohexylcarbodiimide (DCC) (0.50 mmol, 103 mg), and finally 1 mL of NMP was added as a solvent, the reactor was sealed, and reacted at 110° C. for 24 h. After the reaction system was cooled, 2 mL of water and 5 mL of ethyl acetate were added and stirred for 30 min, then extracted three times with 15 mL of ethyl acetate, and the organic phases were combined. The organic phase was washed with saturated brine, and finally dried with anhydrous magnesium sulfate for 2 h. After filtration, the organic phase was rotary evaporated to obtain a crude product. The crude product was separated by column chromatography with petroleum ether: ethyl acetate=3:1 (v / v) as the eluent and silica gel as the adsorption phase to obtain a light brown sol...
Embodiment 3
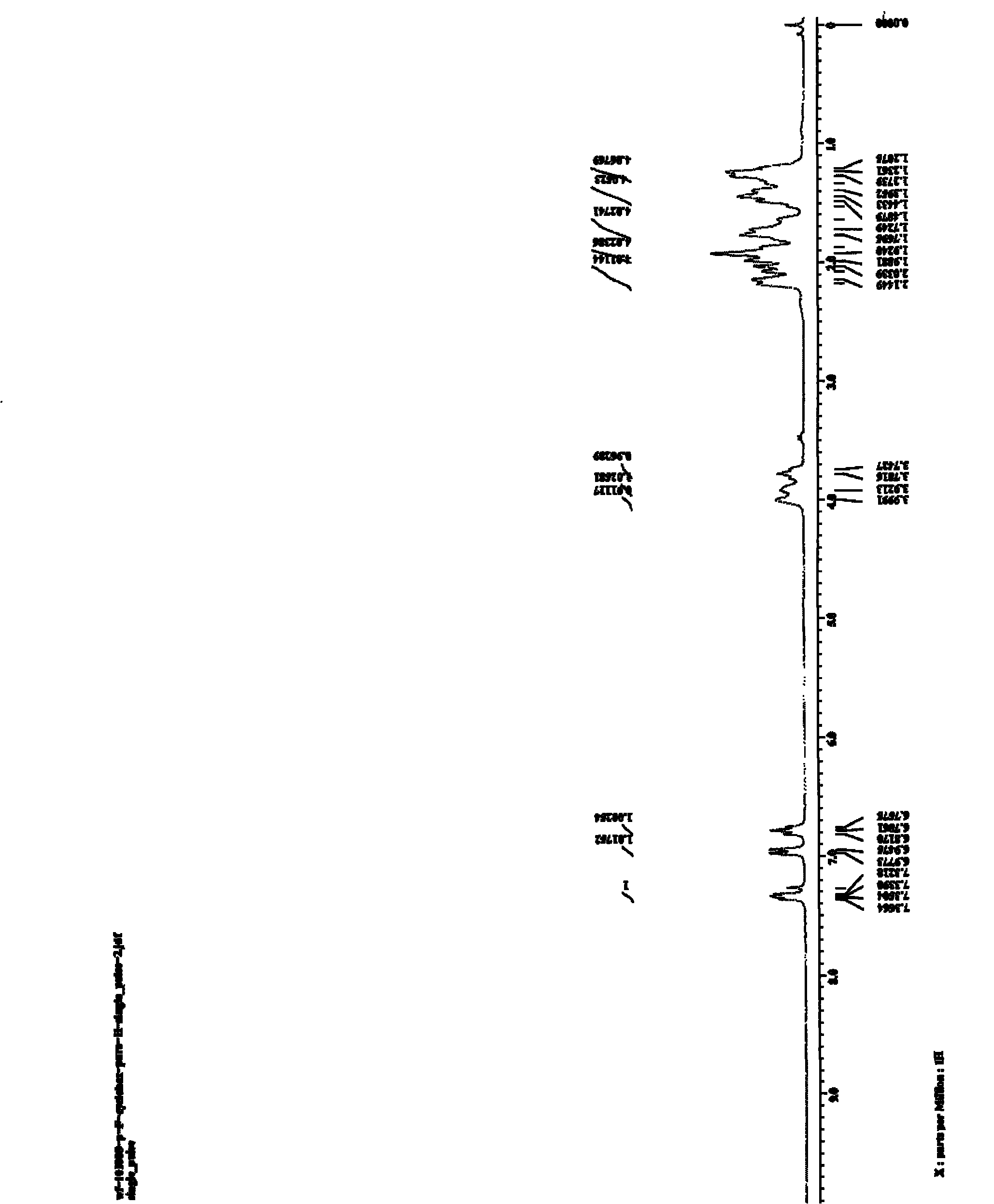
[0031] Embodiment 3, N, the preparation of 1-dicyclohexyl-6-fluoro-2-aminobenzimidazole
[0032] To a 20mL reactor, add 4-fluoro-o-bromoaniline (0.50mmol, 57μL), CuI (0.05mmol, 10mg), sodium tert-butoxide (1.0mmol, 96mg), N,N-dicyclohexylcarbodi Dicyclohexylcarbodiimide (DCC) (0.50 mmol, 103 mg), and finally 1 mL of NMP was added as a solvent, the reactor was sealed, and reacted at 110° C. for 16 h. After the reaction system was cooled, 2 mL of water and 5 mL of ethyl acetate were added and stirred for 30 min, then extracted three times with 15 mL of ethyl acetate, and the organic phases were combined. The organic phase was washed with saturated brine, and finally dried with anhydrous magnesium sulfate for 2 h. After filtration, the organic phase was rotary evaporated to obtain a crude product. The crude product was separated by column chromatography with petroleum ether: ethyl acetate=3:1 (v / v) as the eluent and silica gel as the adsorption phase to obtain a light brown soli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com