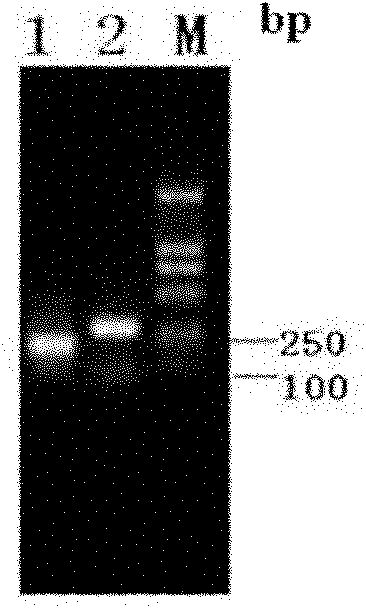Fusion expression product of antimicrobial peptide genes of two marine animals and preparation method of fusion expression product
A technology that integrates expression and marine animals, applied in the biological field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
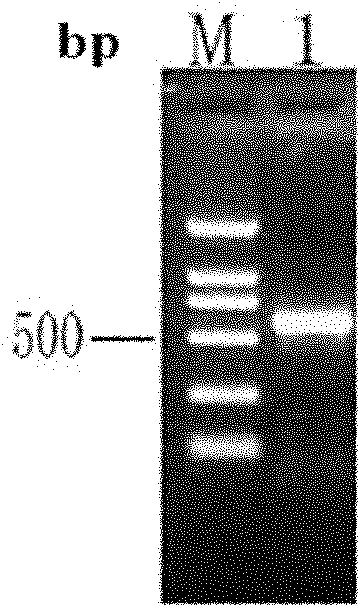
[0067] Example 1 Construction of recombinant expression plasmid pET28a / scygonadin2-hepcidin
[0068] 1) Acquisition of scygonadin2 in Scylla serrata and hepcidin in large yellow croaker:
[0069] According to the multiple cloning sites on the pET28a vector, the scygonadin2 antimicrobial peptide gene of Scylla serrata was designed and synthesized (Genbank accession number: DQ872630 ) and large yellow croaker hepcidin antimicrobial peptide gene (Genbank accession number: EF156401 ) specific upstream and downstream primers. Add an NcoI restriction site to the 5′ end of the upstream primer of the Scylla serrata scygonadin2 gene, and the upstream primer F1 is:
[0070] F1: 5′CAC CCATGG CGAATGGCCTGGCACTCAACAGGCTTATG 3′
[0071] Wherein the black italics indicate the introduced NcoI restriction site.
[0072] Mutate the XhoI restriction site at the 5′ end of the downstream primer of the scygonadin2 antimicrobial peptide gene of Scylla serrata, add a connecting peptide gene se...
Embodiment 2
[0140] Example 2 Induced expression of pET28a / scygonadin2-hepcidin recombinant plasmid in Escherichia coli
[0141]1) Induced expression: Extract the correct identified pET28a / scygonadin2-hepcidin recombinant plasmid by alkaline lysis method, and transform pET28a empty plasmid and pET28a / scygonadin2-hepcidin recombinant plasmid into E.coli BL21(DE3) competent cells by heat shock method in LB solid medium plate containing kanamycin (50 μg / ml), cultured overnight at 37°C; the next day, pick out several pET28a empty plasmids and pET28a / scygonadin2-hepcidin recombinant plasmids to transform expression strains and grow Single colonies were inoculated in 20ml LB liquid medium (containing 50μg / ml kanamycin), shaken at 200rpm, 37°C until OD 600 About 0.3 (OD 600 is the absorbance value of the bacterial culture solution at 600nm), then IPTG was added to a final concentration of 0.6mmol / L, and cultured with shaking at 180rpm at 28°C for 5h.
[0142] 2) Detection of fusion protein expr...
Embodiment 3
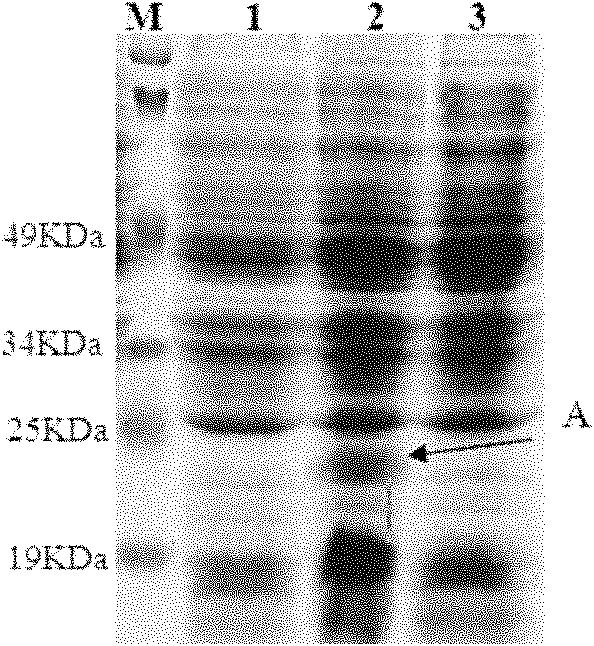
[0143] Example 3 Solubility analysis of expression products of pET28a / scygonadin2-hepcidin recombinant plasmid in Escherichia coli
[0144] 1) Transform and culture 20ml of seed solution as above, take 2ml of seed solution and add it to 200ml of LB liquid medium containing 50μg / ml kanamycin, and culture on a shaker at 200rpm at 37°C for 1.5-2h to OD 600 About 0.3, add IPTG to a concentration of 0.6mmol / L, and induce at 28°C for 5h on a shaker at 180rpm.
[0145] 2) Collect the cells by centrifugation, suspend the cells in 20 ml of pre-cooled PBS (pH7.4), freeze and thaw once at -20°C, and disrupt the cells of the cells by ultrasonic in an ice bath.
[0146] 3) Centrifuge the above-mentioned sonicated liquid at 4°C, 12000g, for 15min. The supernatant and the precipitate were collected respectively, and SDS-PAGE electrophoresis was carried out as described above to confirm that the target protein was expressed in the form of inclusion bodies (see Figure 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com