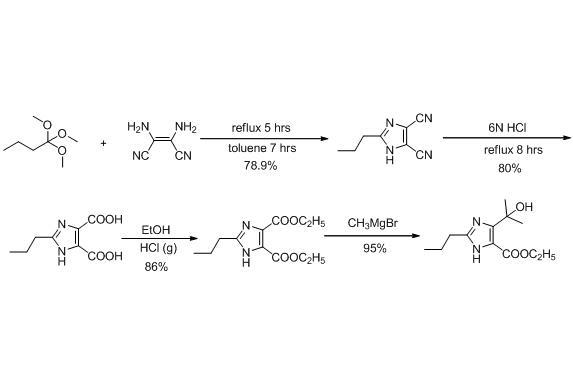
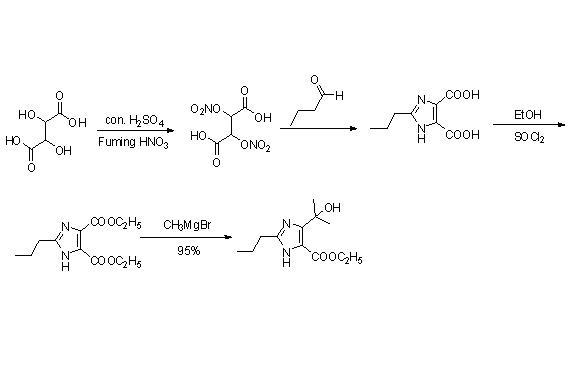
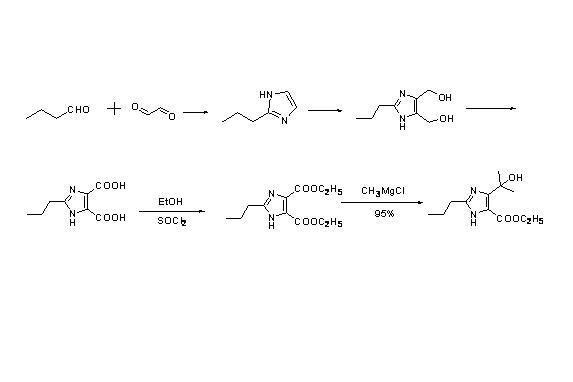New method for synthesizing 4-(1-hydroxyl-1-methyl ethyl)-2-propyl imidazole-5-carboxylic acid ethyl ester
A technology of dimethylolimidazole and propylimidazole, applied in the direction of organic chemistry and the like, can solve the problems of low boiling point of diethyl ether, difficult to control reaction temperature, easy volatilization and the like, and achieves the effects of mild reaction conditions, low production cost and easy purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0027] step 1:
[0028] Preparation of 2-n-Propylimidazole
[0029] Add water (100ml) and ammonium bicarbonate (158.1g, 2.0mol) to the 1000ml reaction bottle, start stirring, and cool down to 0~10 o C. Add n-butyraldehyde (72.11g, 1.0mol) dropwise, and stir for 1 hour after the dropwise addition, and then add 40% aqueous solution of glyoxal (145.1g, 1.0mmol) dropwise. Temperature control 15~25 o C, reacted for 24 hours, added 300ml of ethyl acetate for extraction and separation, the aqueous phase was extracted with 200ml of ethyl acetate, and the organic phase was concentrated under reduced pressure to obtain 90g of brown oil. The crude product yield: 81.8%, HPLC: 91%.
[0030] Step 2:
[0031] Preparation of 2-n-Propyl-4,5-Dihydroxymethylimidazole
[0032] In reaction bottle, add 37% formaldehyde aqueous solution, crude product 90g in above-mentioned example 1, be warming up to 60~70 o C, add 40% aqueous potassium hydroxide solution dropwise, adjust the pH of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com