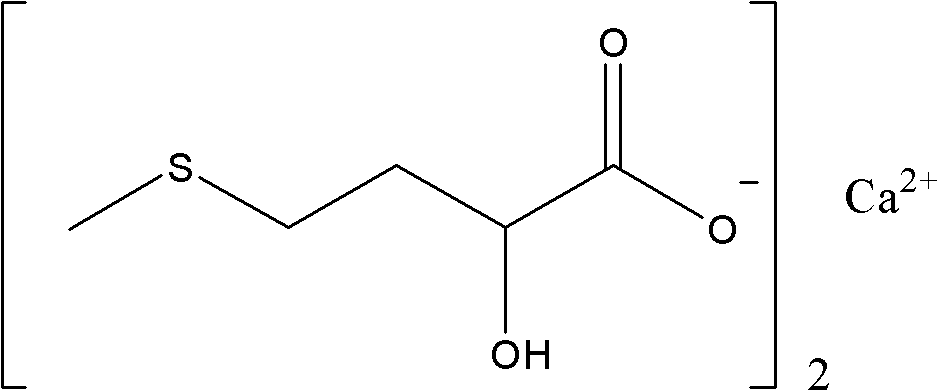
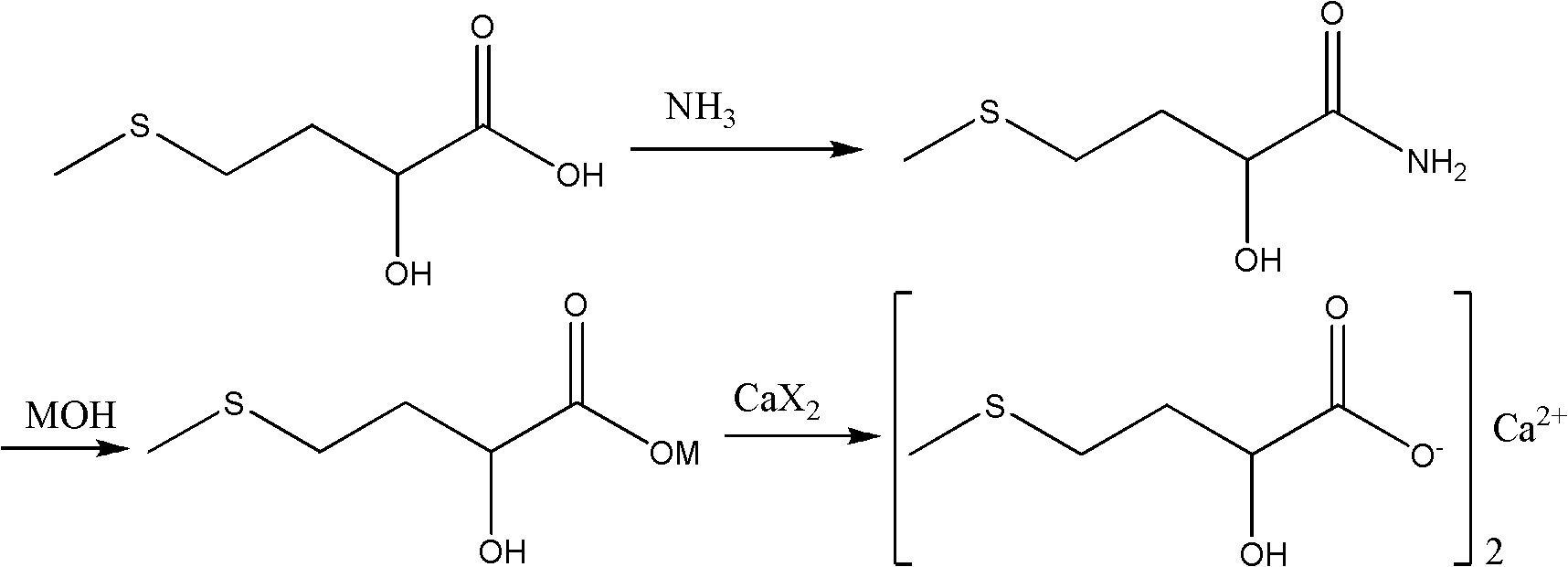
Synthesis method of medicinal calcium D,L-2-hydroxyl-4-(methylthio)butyrate
A technology of calcium methylthiobutyrate and methylthiobutyric acid, applied in the field of medicinal D, can solve the problems of difficult drying of products, low product yield, harsh conditions, etc., and achieves environmentally friendly, easy purification, and mild process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: D, the preparation of L-2-hydroxyl-4-methylthiobutyramide
[0028] In a 500mL four-necked flask with mechanical stirring and a thermometer, drop into a content of 85% D, 176.5g (1.0mol) of L-2-hydroxyl-4-methylthiobutyric acid, stir, and cool in an ice-water bath at 20 102.0 g (1.2 mol) of 20% ammonia water was added dropwise at -30°C. After the dropwise addition was completed, the mixture was stirred at room temperature for 0.5 hour, then heated to 50-60°C and stirred for 2 hours. Evaporate water under reduced pressure and vacuum, keep the vacuum degree of 200Pa and raise the temperature, gradually raise the temperature to 150°C, dehydration reaction at 150-160°C for 6 hours, and cool to room temperature. Add 500 mL of dichloromethane for extraction, add 5 g of activated carbon to the dichloromethane layer, decolorize and filter. The dichloromethane extract was evaporated to dryness and recrystallized with 200 mL of n-hexane to obtain 121.0 g of D,L-2-hy...
Embodiment 2
[0029] Embodiment 2: D, the preparation of L-2-hydroxyl-4-methylthiobutyramide
[0030] In a 500mL four-necked flask with mechanical stirring and a thermometer, drop into a content of 85% D, 176.5g (1.0mol) of L-2-hydroxyl-4-methylthiobutyric acid, stir, and cool in an ice-water bath at 20 102.0 g (1.2 mol) of 20% ammonia water was added dropwise at -30°C. After the dropwise addition was completed, the mixture was stirred at room temperature for 0.5 hour, then heated to 50-60°C and stirred for 2 hours. Evaporate water under reduced pressure and vacuum, keep the vacuum degree of 200Pa and raise the temperature, gradually raise the temperature to 150°C, dehydrate at 170-180°C for 4 hours, and cool to room temperature. Add 500 mL of dichloromethane for extraction, add 5 g of activated carbon to the dichloromethane layer, decolorize and filter. The dichloromethane extract was evaporated to dryness, and recrystallized with 200 mL of n-hexane to obtain 129.0 g of D,L-2-hydroxy-4-me...
Embodiment 3
[0031] Embodiment 3: D, the preparation of L-2-hydroxyl-4-methylthiobutyrate calcium
[0032] In a 500mL four-neck flask with mechanical stirring and a thermometer, put 98.5% D, L-2-hydroxyl-4-methylthiobutyramide 75.6g (0.5mol), and add 30% sodium hydroxide 80.0g (0.6mol), heated up to 100-105°C and stirred for 2 hours, it was detected that the conversion of D,L-2-hydroxy-4-methylthiobutyramide was completed, the temperature was lowered to 60°C, and 30 111.0 g (0.3 mol) of aqueous calcium chloride solution, after the dropwise addition, continue to stir and react at 50-60° C. for 1 hour, and cool to room temperature. Filter, wash with water, and recrystallize the filter cake with 20% aqueous ethanol to obtain 70.1 g of calcium D,L-2-hydroxy-4-methylthiobutyrate, with a content of 99.3% and a yield of 82.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com