Method for synthesizing alkyl tetrahydrofurfuryl ether and sodium tetrahydrofurfuryl alcoholate
A technology of alkyl tetrahydrofurfuryl ether and tetrahydrofurfuryl alcohol, which is applied in the synthesis of asymmetric ether compounds, the synthesis of alkyl tetrahydrofurfuryl ether and the by-product of sodium tetrahydrofurfuryl alcohol, and can solve the problem of intractable lye and ethyl bromide. alkane leakage, environmental pollution and other problems, to achieve the effect of reducing pollution emissions, reducing production costs and eliminating risk factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] In a 1L four-port reactor, add 2mol tetrahydrofurfuryl alcohol, 1.82mol ethyl bromide to react for 0.5 hours under stirring conditions, then add 1.82mol potassium hydroxide, the reaction temperature is 96°C, after reacting for 10 hours, add 1mol Metal sodium, reacted for 10.5 hours, and the reaction temperature was 25°C. Carry out distillation, wash with water, secondary distillation, obtain product 221g, conversion rate is 85%.
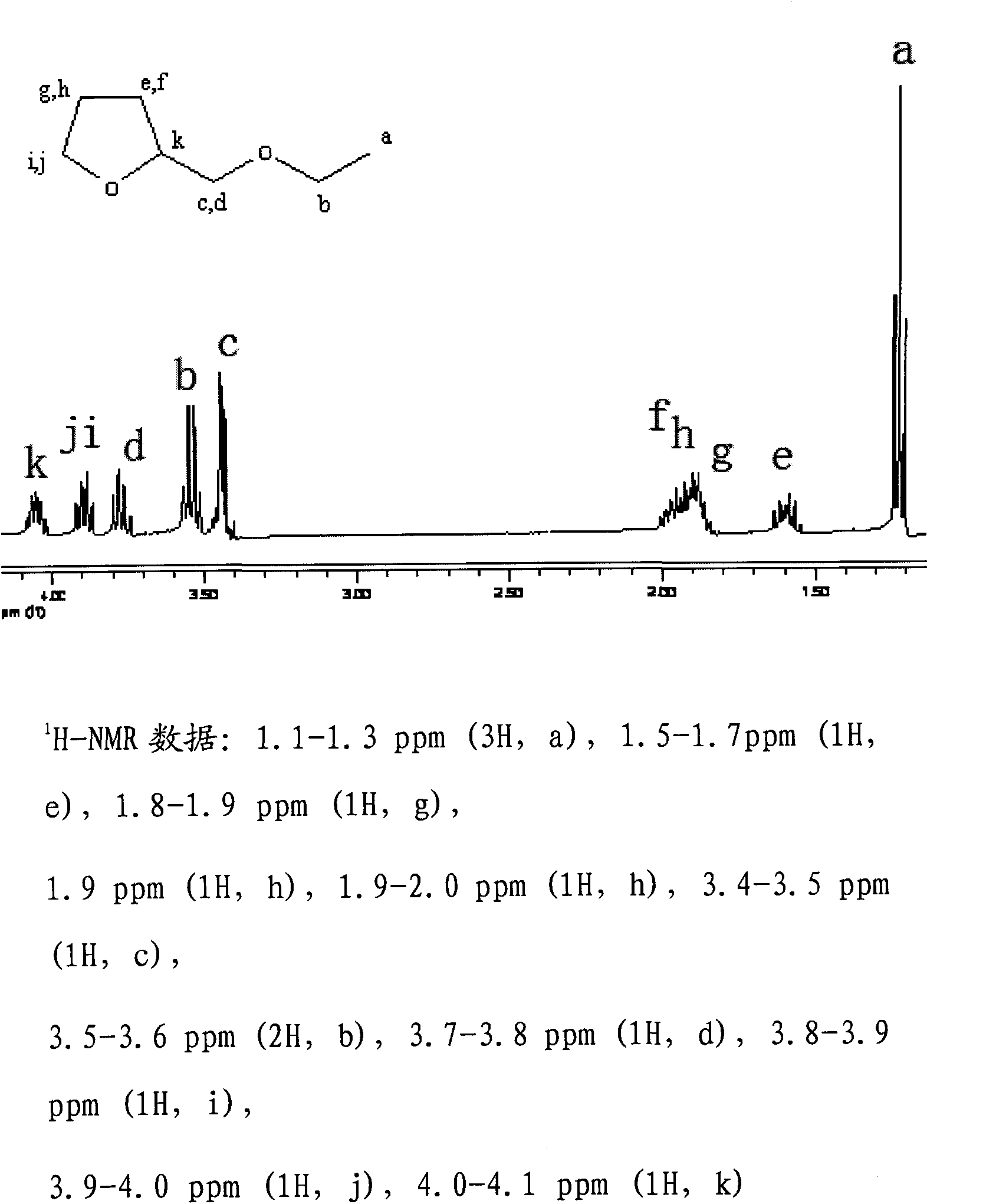
[0041] see Figure 1-3 Shown, with nuclear magnetic, infrared and mass spectrometry, prove that the product of the present embodiment is ethyl tetrahydrofurfuryl ether, product is carried out 1H-NMR is measured, CDCl is solvent, ETE nuclear magnetic spectrum and each hydrogen atom corresponding absorption peak is as shown in the figure shown. It should be noted that there are two absorption peaks for the hydrogen on the tetrahydrofuran ring and the α-position outside the ring.
Embodiment 2
[0043] In a 1L four-port reactor, add 2mol tetrahydrofurfuryl alcohol, 1.9mol ethyl bromide to react for 1.5 hours under stirring conditions, then add 1.9mol potassium hydroxide, the reaction temperature is 120°C, after reacting for 11 hours, add 0.2mol metal sodium, reacted for 11 hours, and the reaction temperature was 30°C. Carried out distillation, washing with water, and secondary distillation to obtain 224 g of ethyl tetrahydrofurfuryl ether product, with a conversion rate of 86%.
Embodiment 3
[0045] In a 1L four-port reactor, add 2mol tetrahydrofurfuryl alcohol, 1.98mol ethyl bromide to react for 3 hours under stirring, then add 1.98mol potassium hydroxide, the reaction temperature is 160°C, after 12 hours, add 0.45mol metal sodium, reacted for 12 hours, and the reaction temperature was 40°C. Distillation, washing with water, and secondary distillation were carried out to obtain 226 g of ethyl tetrahydrofurfuryl ether product, with a conversion rate of 86.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com