Pharmaceutical composition for treating ulcerative colitis and colon-targeted micro-pill preparation thereof
A technology for ulcerative colitis and colon targeting, which is applied in the field of medicine, can solve the problems of affecting the efficacy of drugs and not being able to reach them, and achieve the effects of improving gastrointestinal function, low cost, and promoting the absorption of nutrients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 prepares pharmaceutical composition of the present invention
[0043] 1) Take according to weight ratio: 30 parts of Atractylodes Rhizome, 30 parts of Coptis Rhizoma, and 30 parts of Fangfeng, decoct them into oral solution and pack them separately according to the routine.
[0044] 2) Take respectively according to the weight ratio: 45 parts of Atractylodes Rhizome, 40 parts of Coptis Rhizoma, and 45 parts of Fangfeng, decoct them into oral solution and pack them separately according to the routine.
[0045] 3) Take respectively according to the weight ratio: 55 parts of Atractylodes Rhizome, 40 parts of Coptis Rhizoma, and 60 parts of Fangfeng, decoct them into oral solution and pack them separately according to the routine.
Embodiment 2
[0046] Embodiment 2 prepares a kind of Atractylodes Rhizoma Coptidis pellet preparation
[0047] 1. Preparation of Atractylodes macrocephala volatile oil
[0048] Take 600g of Atractylodes macrocephala, pulverize it into a coarse powder, put it into a supercritical extraction reaction kettle, the extraction temperature is 50°C, the extraction pressure is 35MPa, CO 2 The flow rate is 3L·h -1 , the extraction time was 3h, and the extracted product was collected to obtain 29.7g of Atractylodes macrocephala volatile oil;
[0049] 2. Preparation of Parsnip Extract
[0050] Take 600g of the windproof medicinal material, grind it into coarse powder, put it in an ultrasonic extractor, add 6 times the weight of 75% ethanol aqueous solution, ultrasonically extract twice, 30min each time, filter and combine the extracts, recover the ethanol under reduced pressure and dry it to obtain Wind extract 17.4g;
[0051] 3. Preparation of Cyclodextrin Inclusion Compound of Atractylodes Rhizom...
Embodiment 3
[0065] Embodiment 3 prepares a kind of Atractylodes Rhizoma Coptidis pellet preparation
[0066] The weight ratio of each component of this Atractylodes Rhizoma Coptidis pellet preparation is:
[0067] Atractylodes macrocephala extract: 36 parts,
[0068] Berberine: 7 parts,
[0069] Parsnip Extract: 21 parts.
[0070] The preparation method is the same as in Example 2.
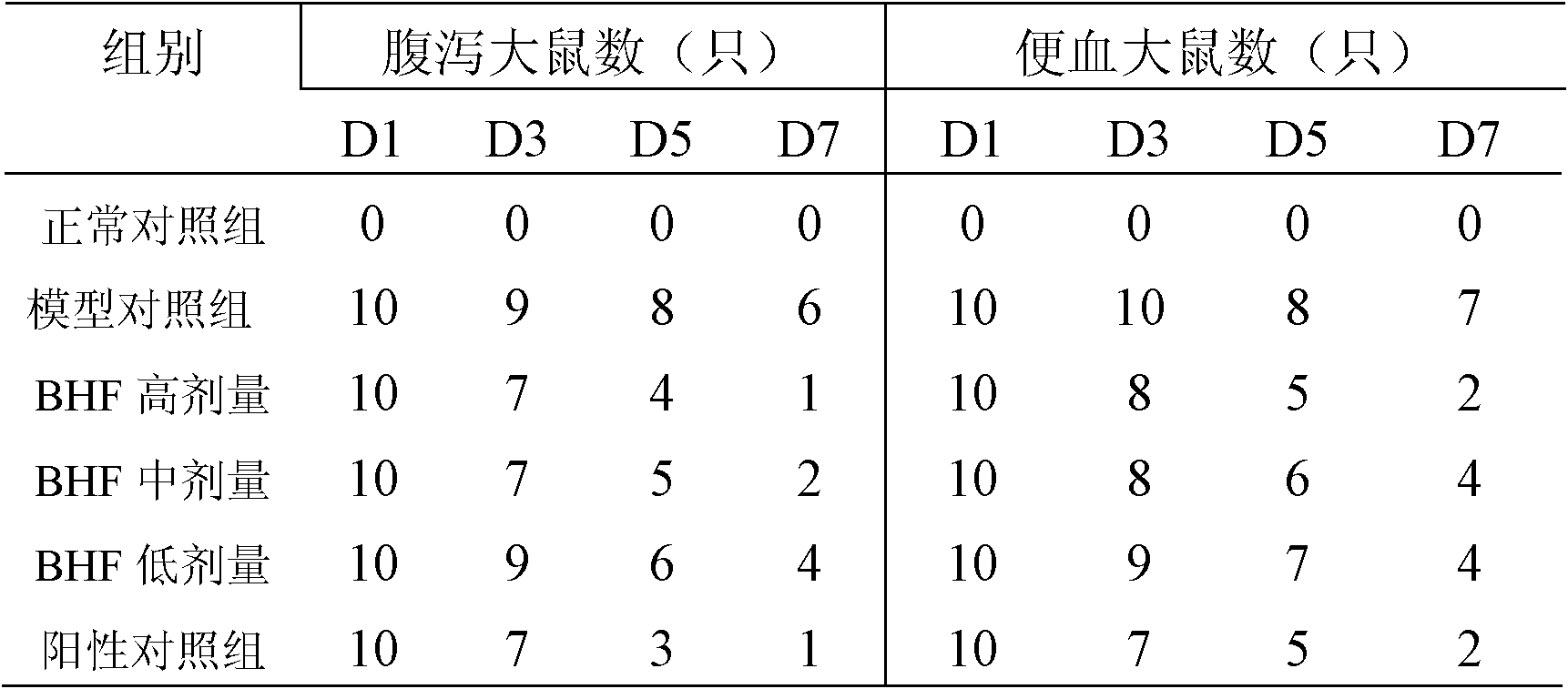
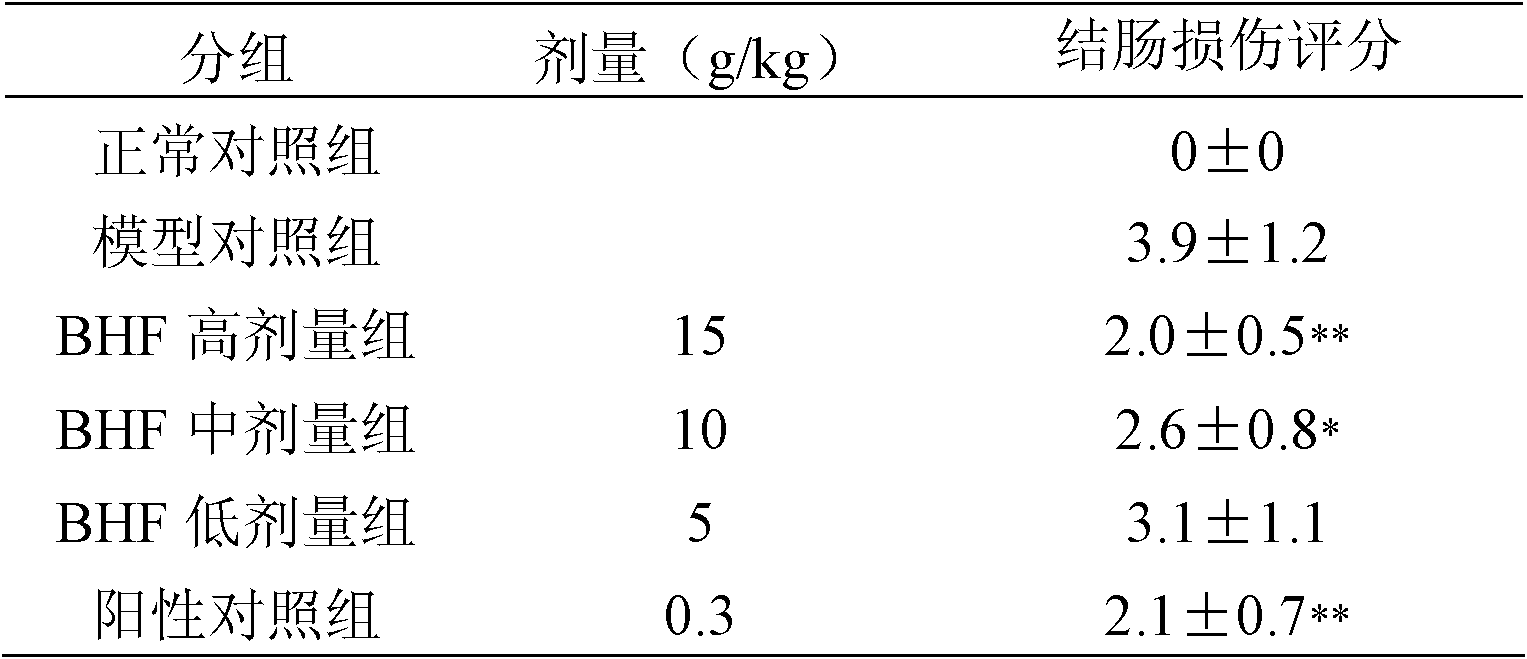
[0071] Drug efficacy evaluation:
[0072] Ulcerative colitis model rats are selected to evaluate the efficacy of the drug of the present invention.
[0073] 1. Experimental animals
[0074] Healthy male SD rats, clean grade, weighing 180g-200g, were purchased from Shanghai Experimental Animal Center, Chinese Academy of Sciences, animal certificate number: [SYXK (Shanghai) 2002-0026], free to eat and drink.
[0075] 2. Test drug
[0076] The pharmaceutical composition of the present invention (abbreviated as BHF) is prepared by (1) of Example 1;
[0077] TNBS was purchased from Sigma Company as a 5% (w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com