Three-dimensional matrices of structured porous monetite for tissue engineering and osseous regeneration, and method for the preparation thereof
A monetite, structured technology, applied in the fields of tissue engineering and bone regeneration, can solve the problems of not being effective and not proposing monetite materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0200] Embodiment 1: the synthetic method of matrix of the present invention
[0201] To synthesize the matrix of the invention, the solid phase was mixed with double distilled water (liquid phase).
[0202] The solid phase includes, but is not limited to, acidic calcium phosphate, basic calcium phosphate, pore inducers such as calcium carbonate, and retarders such as sodium pyrophosphate.
[0203] 1.1 Preparation of solid phase
[0204] The solid phase of the calcium paste consists of alkaline calcium phosphate and acidic calcium phosphate. The basic calcium phosphate is β-tricalcium phosphate (β-TCP), and the acidic calcium phosphate is monocalcium phosphate. These two components were mixed by hand in a mortar and mortar at a molar ratio of 1.785 for 10 minutes. Calcium carbonate is added at a concentration between 1-20% by weight, preferably between 3-10%. 0.54% by weight of sodium pyrophosphate was used as a retarder for the coagulation reaction.
[0205] Specific...
Embodiment 2
[0228] Example 2: Specific production process of specific monetite granules with structured pores
[0229] By way of example and for the purpose of obtaining a viscous slurry with the most preferred characteristics, a powder component formed from 0.8 g anhydrous monocalcium phosphate, 1.4 g β-tricalcium phosphate, 12 mg sodium pyrophosphate and 110 mg calcium carbonate was mixed with 0.77 ml The water is mixed for 30 seconds. One minute after starting to cure, the mold described below was applied to the grout for 30 seconds.
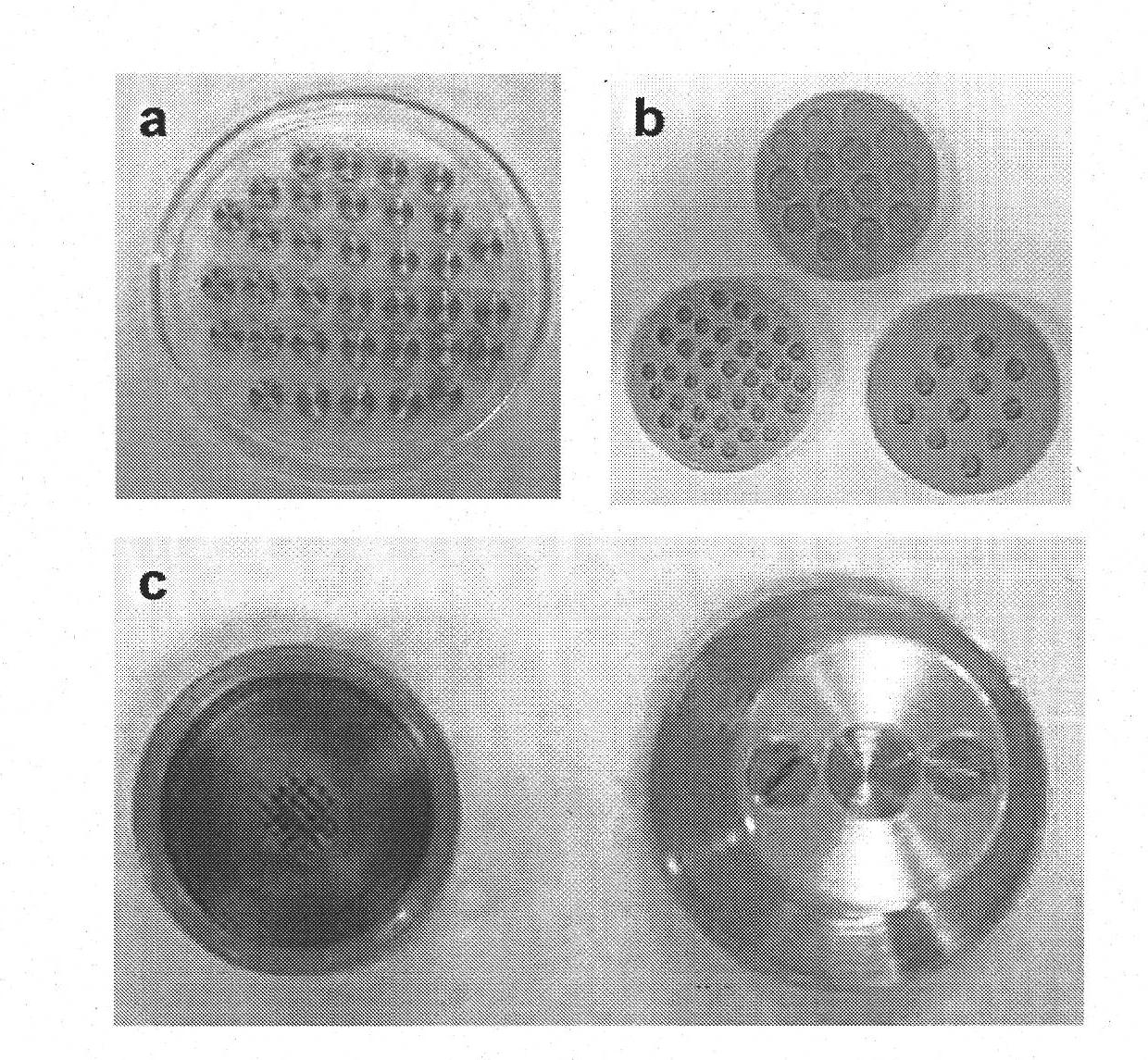
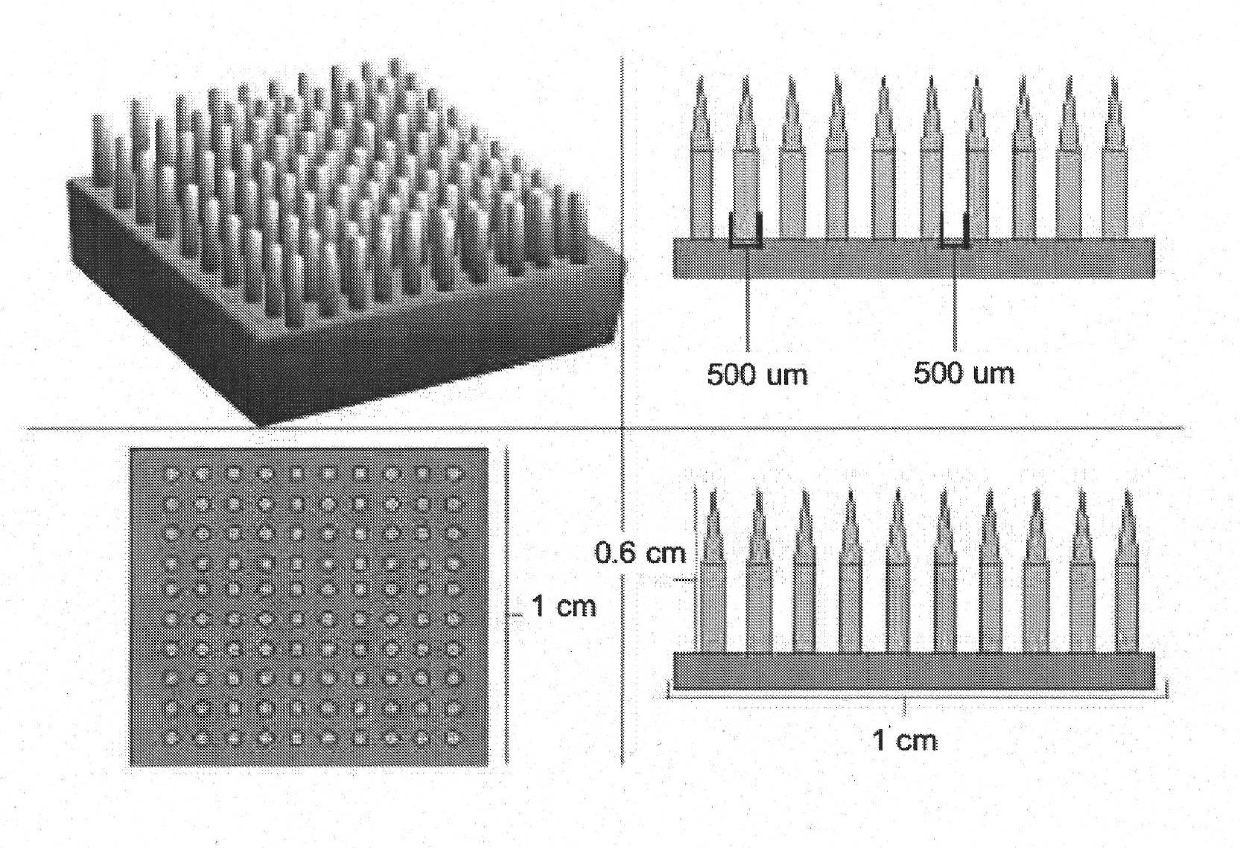
[0230] 2.1 Using a single mold in the described process to obtain a cylindrical matrix with structured porosity
[0231] For the implementation of this example, a silicone mold with the following dimensions and number of punches was used:
[0232] a) Diameter 1cm, height 5mm or 3mm, 64 punching holes
[0233] b) Diameter 0.8cm, height 5mm or 3mm, 39 punching holes
[0234] c) Diameter 0.7cm, height 5mm or 3mm, 28 punching holes
[0235] d) Diame...
Embodiment 3
[0270] Example 3: Between monetite with structured porosity and matrix of amorphous monetite comparative study between
[0271] 3.1 Microscopic research
[0272] A comparison test of the microstructure of the amorphous matrix with that of the matrix with structured porosity was then carried out. To carry out this test, scanning electron microscopy is used by procedures known to those skilled in the art.
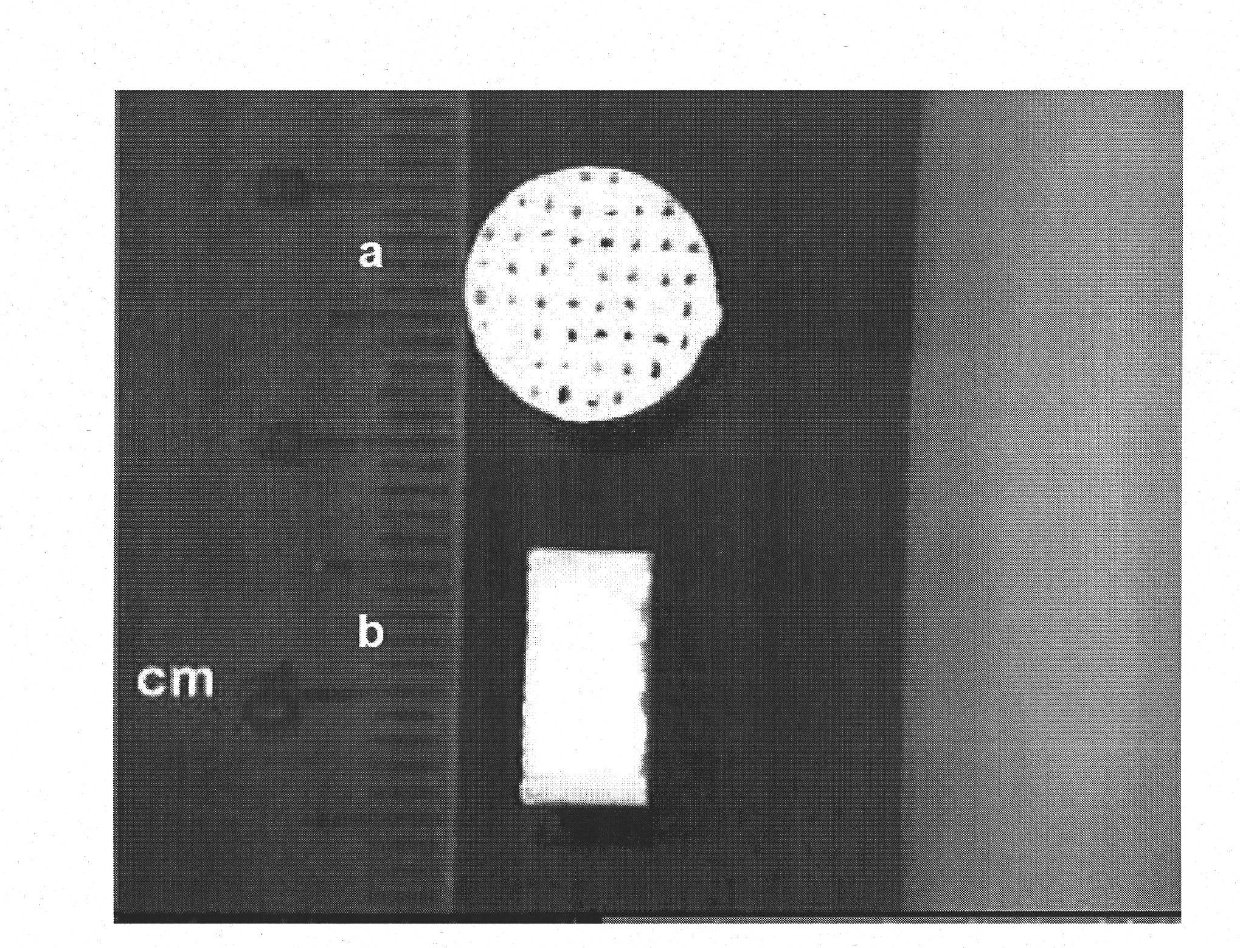
[0273] Microstructure of Amorphous Porous Monetite Matrix
[0274] Biomaterials arranged in the form of an amorphous matrix ( Figure 6 a, b) Obtaining uncontrolled porosity. In other words, they show an irregular distribution of macropores, which is produced during the process of obtaining viscous pulp as described in Examples 1.1 to 1.6. The macropores of the amorphous matrix are cavities in the biomaterial and do not connect internal structures ( Figure 7 ).
[0275] With regard to the number and distribution of macropores, their absence is observed. The pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com