Synthesis method of azoic coupling component AS-D series pigment for aqueous application system and product obtained by synthesis method
A synthesis method and chromophenol technology, applied in the application, chemical instruments and methods, azo dyes and other directions, can solve the problems that do not involve the influence of product crystals, environmental pollution, process complexity, etc., and achieve bright colors, easy dispersion, simple craftsmanship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
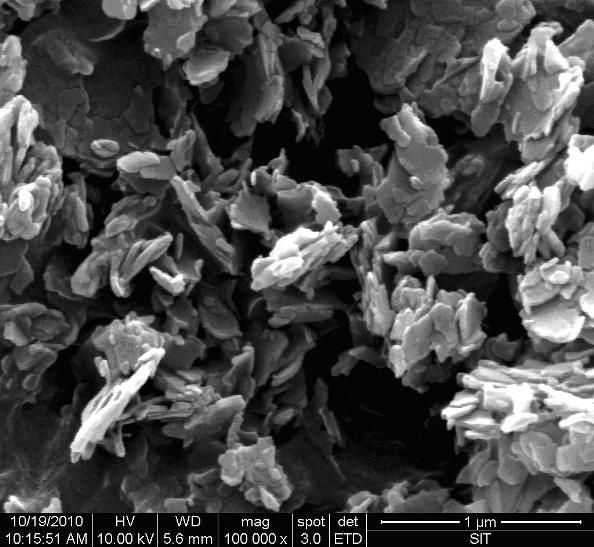
Image
Examples
Embodiment 1
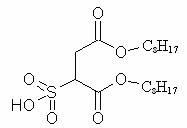
[0033] Example 1 Synthesis of C.I. Pigment Red 112 Using Sulfonated Succinic Acid Surfactant IIB
[0034] The structure of sulfosuccinic acid surfactant IIB is as follows:
[0035]
[0036] Preparation of 2,4,5-trichloroaniline diazonium salt solution: Add 49.0g 2,4,5-trichloroaniline (0.25mol) and 150ml of water into a large beaker, stir for 40 minutes to fully moisten the trichloroaniline wet. Add 150 g of 30% hydrochloric acid again, and continue stirring for 8 hours. Add crushed ice to cool it down to -5 to 0°C, quickly add 53.0g (35%, 0.27mol) of sodium nitrite solution under liquid, and stir for 60 minutes. If necessary, add additional sodium nitrite or sulfamic acid to ensure a slight excess of sodium nitrite. Filter and adjust the volume of the solution to 800ml, and ensure that the temperature of the diazo solution is between 0 and 5°C by adding ice.
[0037] Preparation of naphthol AS-D coupling component suspension: Add 70.0g of 30% liquid caustic soda and...
Embodiment 2
[0042] Example 2 Synthesis of C.I. Pigment Red 112 Using Sulfonated Succinic Acid Surfactant IID
[0043] The structure of sulfonated succinic acid surfactant IID is as follows:
[0044]
[0045] According to the process synthesis conditions of Example 1, C.I. Pigment Red 112 was synthesized as the sample of Example 2 with sulfosuccinic acid surfactant IID instead of sulfosuccinic acid surfactant IIB.
[0046] The sample of Example 2 was pressed into tablets with KBr, and the infrared absorption spectrum was measured and compared with the infrared spectrum of permanent red FGR. Strong absorption (1673, 1592, 1552 and 1484 cm -1 ) and absorption in the fingerprint region (1250, 1203, 1159, 1011, 750, 516 cm -1 ) is completely consistent with the spectrum of Everlasting Red FGR. By color measurement, the coloring power of the sample of Example 2 in polyacrylic resin is 12% higher than that of permanent red FGR.
Embodiment 3
[0047] Example 3 Sulfonated succinic acid surfactant IIB dosage experiment
[0048] According to the synthesis conditions of Example 1, 11.3g (42%, 0.01mol) of sulfosuccinic acid surfactant IIB was used to synthesize C.I. Pigment Red 112 as the sample of Example 3-1. Through color measurement, the coloring power in polyacrylic resin is 4% higher than that of permanent red FGR.
[0049] According to the synthesis conditions of Example 1, 70.5g (42%, 0.062mol) of sulfosuccinic acid surfactant IIB was used to synthesize C.I. Pigment Red 112 as the sample of Example 3-2. The sample can be instant dissolved in polyacrylic resin after milling. Although the tinting strength is lower than that of permanent red FGR when diluting the color measurement due to the high content of surfactant, the natural color concentration is equivalent to that of permanent red FGR. The advantage is that it can be used as an instant pigment in water-based concentrated color systems without pre-dispersi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com