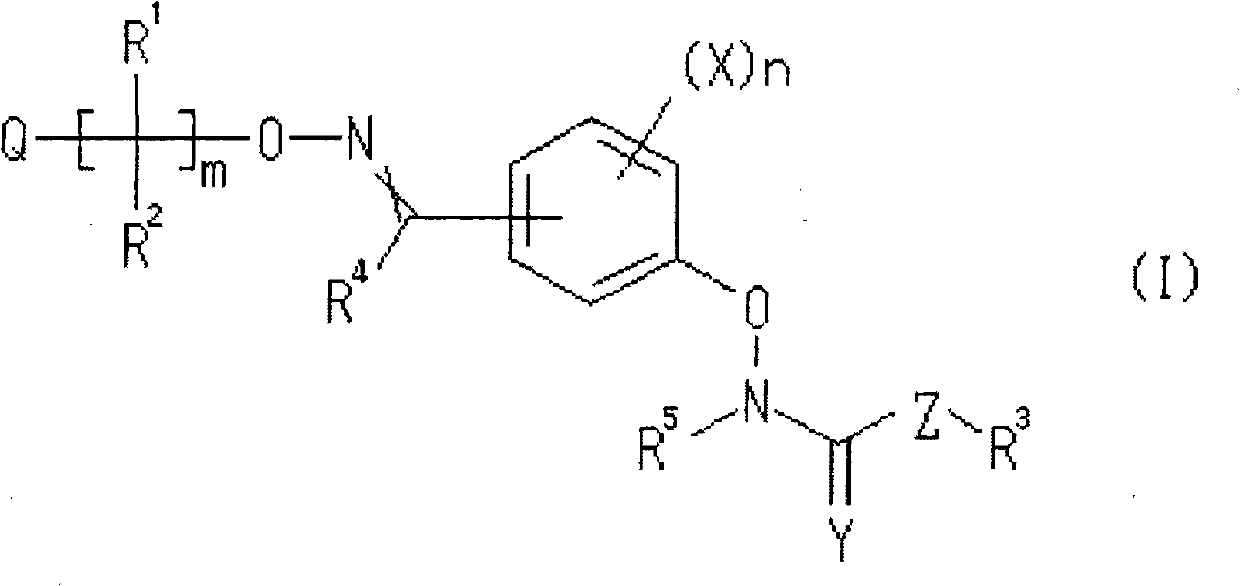
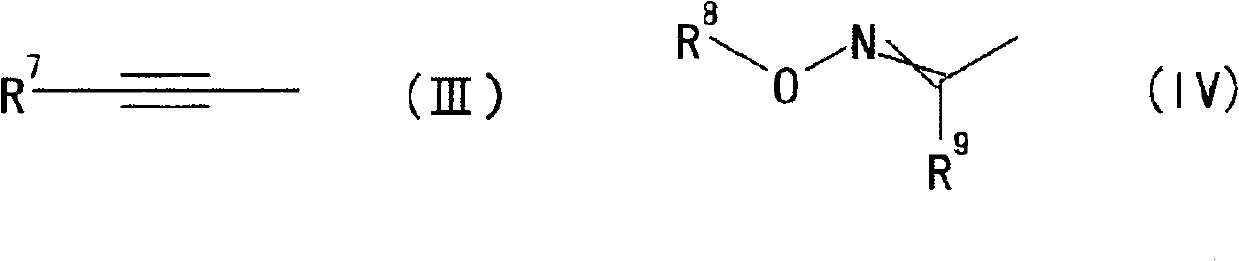
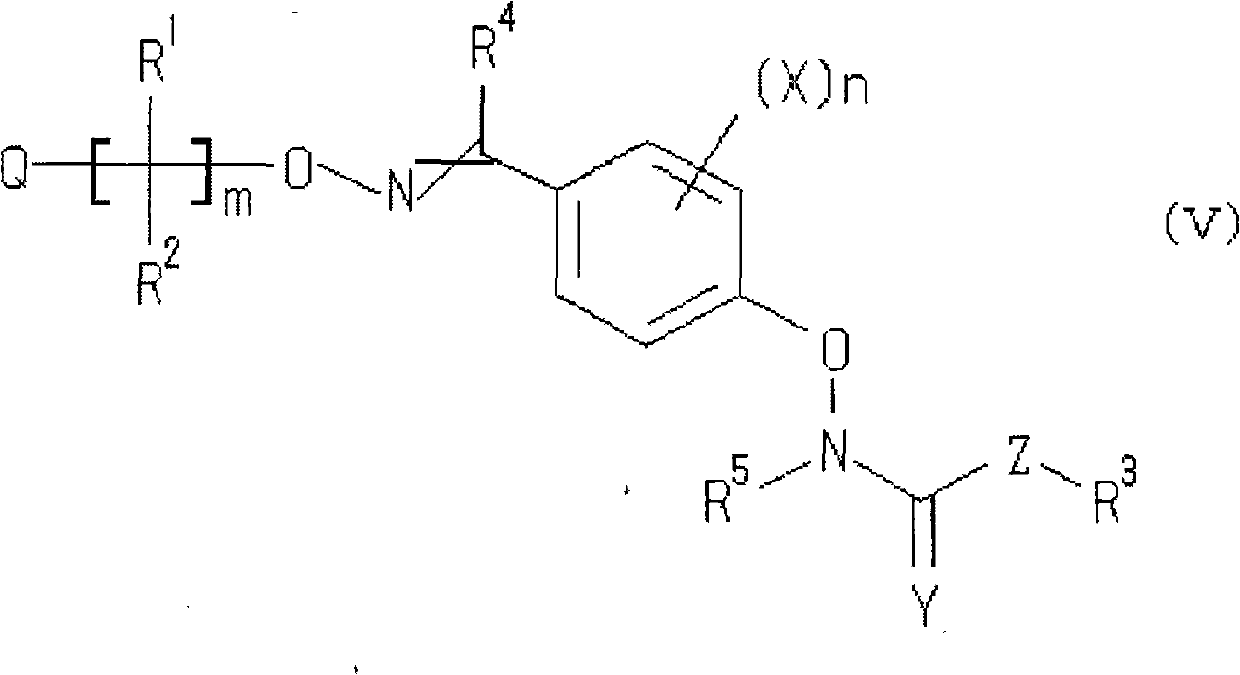
Oxime ether derivative and bactericide for agricultural and horticultural use
A kind of technology of derivatives and oxime ethers, applied in the field of oxime ether derivatives and their salts, and agricultural and horticultural fungicides, can solve the problems such as no compounds are given, manufacture examples and examples are not specifically recorded, and the effect is reliable and excellent. Control effect, toxicity and effect with low environmental impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0500] Production of methyl N-(2-chloro-5-{1-[3-fluoro-2-(trifluoromethyl)benzyloxyimino]ethyl}phenoxy)carbamate (compound 1-13)
[0501] (Process 1)
[0502] Production of tert-butyl N-[3-fluoro-2-(trifluoromethyl)benzyloxy]carbamate
[0503]
[0504] Add 20ml of acetonitrile, 0.63g of N-tert-butoxycarbonyl hydroxylamine, 1,8-diazabicyclo[5.4.0]-7-deca to 1.00g of 3-fluoro-2-(trifluoromethyl)benzyl bromide Monocarbene (DBU) 0.72g, stirred at room temperature for 3 hours. After completion of the reaction, ethyl acetate was added, followed by washing with 7% hydrochloric acid and 10% aqueous sodium hydroxide solution in this order. The obtained organic layer was dried over magnesium sulfate, filtered, and the solvent was distilled off under reduced pressure to obtain 1.20 g of the target crude N-[3-fluoro-2-(trifluoromethyl)benzyloxy]carbamate tert-butyl ester .
[0505] (Process 2)
[0506] Production of methyl N-(2-chloro-5-{1-[3-fluoro-2-(trifluoromethyl)benzyloxyimi...
Embodiment 2
[0510] Production of methyl N-{2-chloro-5-[1-(2-iodobenzyloxyimino)ethyl]phenoxy}carbamate (compound 11-9)
[0511] (Process 1)
[0512] Production of tert-butyl N-(2-iodobenzyloxy)carbamate
[0513]
[0514] Add 20 ml of acetonitrile, 0.55 g of N-tert-butoxycarbonyl hydroxylamine, and 0.62 g of 1,8-diazabicyclo[5.4.0]-7-undecene (DBU) into 1.00 g of 2-iodobenzyl bromide, Stir at room temperature for 1 hour. After completion of the reaction, ethyl acetate was added, followed by washing with 7% hydrochloric acid and 10% aqueous sodium hydroxide solution in this order. The obtained organic layer was dried over magnesium sulfate, filtered, and the solvent was distilled off under reduced pressure to obtain 1.08 g of the target crude tert-butyl N-(2-iodobenzyloxy)carbamate (crude yield 92%).
[0515] (Process 2)
[0516] Production of methyl N-{2-chloro-5-[1-(2-iodobenzyloxyimino)ethyl]phenoxy}carbamate
[0517]
[0518] A mixture of 0.30 g of methyl N-(5-acetyl-2-chloro...
Embodiment 3
[0520] Production of methyl N-{2-methyl-5-[1-(2-trifluoromethylbenzyloxyimino)ethyl]phenoxy}carbamate (compound 21-24)
[0521] (Process 1)
[0522] Production of 4-bromo-2-(4-methoxybenzyloxy)-1-methylbenzene
[0523]
[0524] 30.0 g of 4-bromo-2-fluorotoluene and 23.1 g of p-methoxybenzyl alcohol were dissolved in 240 ml of DMI (dimethylimidazolinone), and 6.68 g of sodium hydride was added under ice cooling, and stirred at 110°C for 1 hour. After cooling to room temperature, the reaction solution was poured into aqueous ammonium chloride solution, and extracted with ethyl acetate. Magnesium sulfate was added to the organic layer to dry, and after filtration, the solvent was distilled off under reduced pressure, and the resulting residue was purified by silica gel column chromatography (developing solvent: hexane:ethyl acetate=9:1) to obtain the target 4-bromo- 42.8 g of 2-(4-methoxybenzyloxy)-1-methylbenzene (88% yield).
[0525] (Step 2) Production of 1-[3-(4-methoxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com