Mayenite-type compound and process for production of same
A technology of mayenite and compounds, which is applied in the field of mayenite-type compounds and its manufacture, can solve the problems of difficult long-term storage, deterioration of secondary electron emission coefficient and other characteristics, achieve chemical stability, excellent oxidation resistance, high productivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
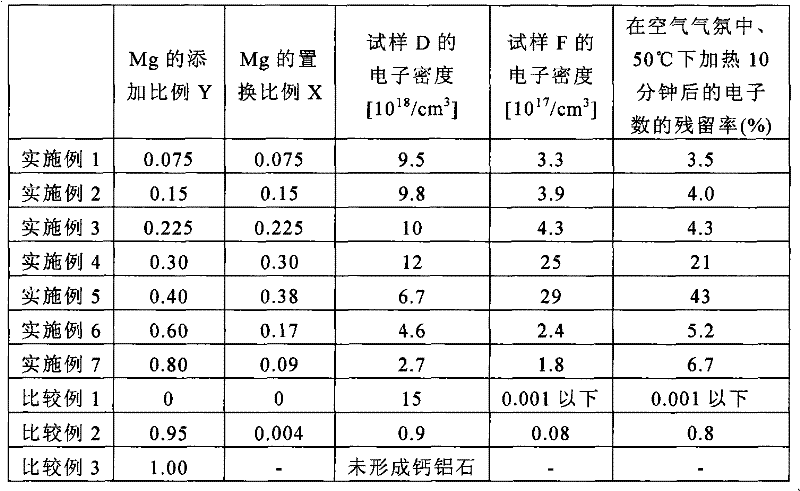
[0119] Powdered calcium carbonate (CaCO 3 ), magnesium oxide (MgO) and aluminum oxide (Al 2 o 3 ) were mixed at a molar ratio of 11.1:0.9:7 (=12(1-0.075):12×0.075:7) (addition amount Y=0.075).
[0120] In addition, here, in addition to calcium carbonate, magnesia, and alumina, ion-exchanged water and a dispersant (trade name: BYK180, manufactured by Bicchem Co., Ltd.) were further added and mixed. When the total of calcium carbonate, magnesia, and alumina is 100 parts by mass, the amounts added here are 100 parts by mass of ion-exchanged water and 0.30 parts by mass of the dispersant. Mix using a ball mill and mix for 3 hours.
[0121] Then, the obtained mixed powder was held in a drier (100° C.) for 6 hours to be dried.
[0122]Then, the dried mixed powder is put into an alumina crucible and calcined in an air atmosphere. Here, after heating up to 1100° C. at a heating rate of 300° C. / hour, the solid-state reaction was carried out by holding for 2 hours, and then slowly ...
Embodiment 2
[0132] Powdered calcium carbonate (CaCO 3 ), magnesium oxide (MgO) and aluminum oxide (Al 2 o 3 ) were mixed in a molar ratio of 10.2:1.8:7 (=12(1-0.15):12×0.15:7) (addition amount Y=0.15), except that, the same operation as Example 1 was performed to obtain a white Crystalline calcined powder A2. Then, it carried out similarly to Example 1, and obtained samples B2-F2 from calcined powder A2.
[0133] The electron densities of samples D2 and F2 can be seen from the ESR signals to be 9.8×10 18 / cm 3 and 3.9×10 17 / cm 3 . The electron density ratio of the sample D2 which is the sample before the heat treatment and the sample F2 which is the sample after the heat treatment is 0.04 times. That is, it can be seen that 4% of electrons remain by heat treatment, and it has high oxidation resistance.
Embodiment 3
[0135] Powdered calcium carbonate (CaCO 3 ), magnesium oxide (MgO) and aluminum oxide (Al 2 o 3 ) were mixed in a molar ratio of 9.3:2.7:7 (=12(1-0.22):12×0.22:7) (addition amount Y=0.22), except that, the same operation as in Example 1 was performed to obtain a white Crystalline calcined powder A3. Then, it carried out similarly to Example 1, and obtained samples B3-F3 from calcined powder A3.
[0136] The electron densities of samples D3 and F3 can be seen from the ESR signals to be 1.0×10 19 / cm 3 and 4.3×10 17 / cm 3 . The electron density ratio of the sample D3 which is the sample before the heat treatment and the sample F3 which is the sample after the heat treatment was 0.04 times. That is, it can be seen that 4% of electrons remain by heat treatment, and it has high oxidation resistance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electron density | aaaaa | aaaaa |
| Electron density | aaaaa | aaaaa |
| Electron density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com