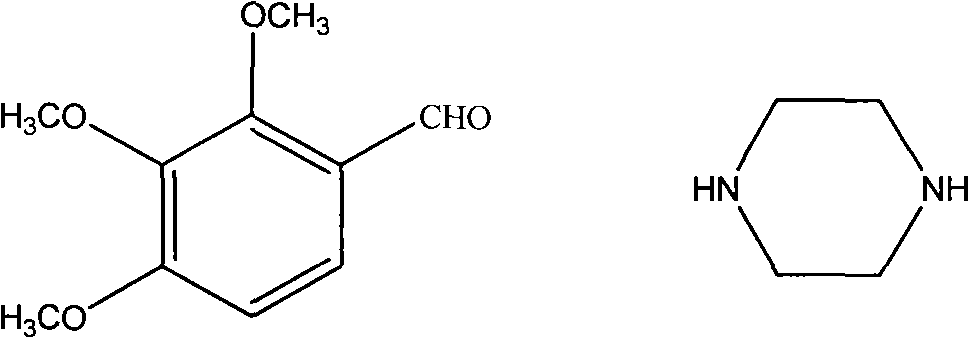
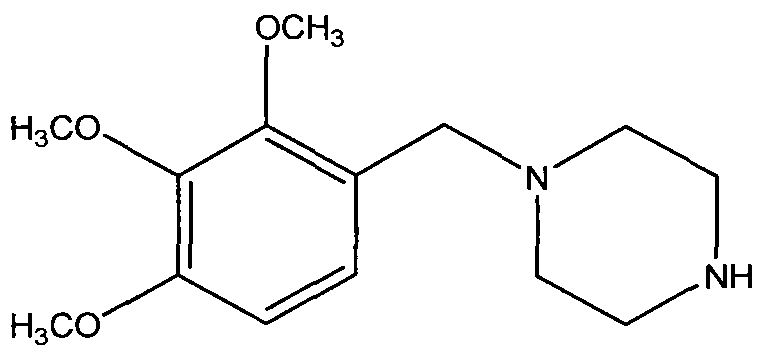
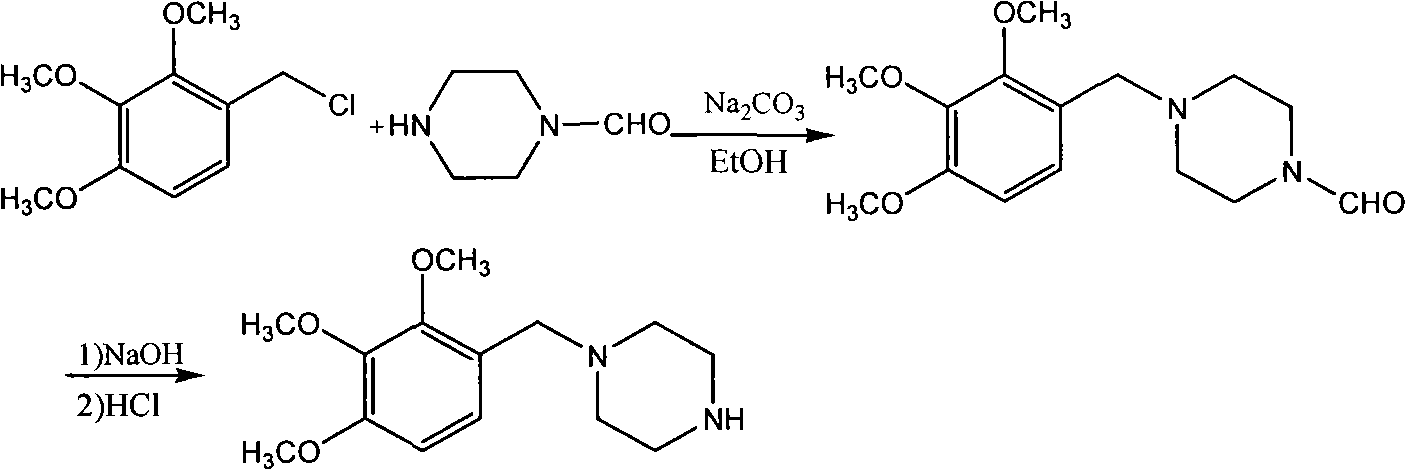
Trimetazidine and production method for hydrochloride of trimetazidine
A technology of trimetazidine and its production method, which is applied in the field of production of trimetazidine and its hydrochloride, can solve the problems of difficult preparation, high risk, unfavorable production, etc., and is beneficial to large-scale production and reduces synthesis Cost, Effect of Enhanced Safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Under the protection of nitrogen, put 78.4g2,3,4-trimethoxybenzaldehyde, 68.8g piperazine, 400mL methyl tert-butyl ether, 4gPd / C into the reactor, quickly raise the temperature of the reaction system to 50-55°C, and then The reaction system was fed with 10 bar hydrogen, and then the temperature was continued to rise to 70°C and maintained at this temperature for about 2 hours, and then the reaction liquid was cooled to 50°C and the catalyst was filtered. Then cool the filtrate to 10°C to filter unreacted piperazine, then add 200mL water to the filtrate, adjust the pH of the filtrate to 7.9-8 with 7N hydrochloric acid at 13-18°C, and add 600mL water to the filtrate, stir for half an hour Separation, the organic phase was extracted twice with 100mL toluene and discarded, the aqueous phase was cooled with an ice-water bath, 42g sodium hydroxide was slowly added, stirred and extracted three times with 120mL toluene, dried over anhydrous magnesium sulfate, and rotary evaporat...
Embodiment 2
[0027] Under the protection of nitrogen, put 78.4g 2,3,4-trimethoxybenzaldehyde, 90.6g piperazine, 400mL ethanol, 4gPd / C into the reactor, quickly raise the temperature of the reaction system to 70°C, and then inject 10bar hydrogen into the reaction system, Then continue to heat up to 70°C and maintain this temperature for about 2 hours, then cool the reaction solution to 20°C and filter the catalyst. Then the filtrate was spin-dried under reduced pressure, added 200mL of toluene cooled to -10°C and stirred, filtered off unreacted piperazine, then added 200mL of water to the filtrate, adjusted the pH of the filtrate to 6 with concentrated hydrochloric acid, and extracted the two parts with 120mL of toluene. Discarded once, the water phase was cooled with an ice-water bath, slowly added 42g of sodium hydroxide, stirred and extracted three times with 120mL of toluene, dried over anhydrous magnesium sulfate, and rotary evaporated under reduced pressure to obtain trimetazidine, yie...
Embodiment 3
[0029] Under nitrogen protection, the trimetazidine 100.1g that example 1 obtains is stirred and dissolved in 216.0g isopropanol, and the filtrate after filtering is transferred to the stainless steel reactor rinsed with isopropanol, and slowly added to fill 79.2g concentrated hydrochloric acid In 348g of isopropanol solution, the temperature was controlled not to exceed 40°C and stirred for half an hour, the reaction solution was distilled to 270g at room temperature and stirred at 0°C for 2 hours, filtered to obtain trimetazidine hydrochloride, and rinsed twice with isopropanol , Yield: 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com