Chromium(iii)-containing aqueous solution and process for production of same
A manufacturing method and aqueous solution technology, applied in the direction of chemical instruments and methods, chromium sulfate, chromium compounds, etc., can solve the problems of poor practicability, difficulty in obtaining a glossy surface, long time, etc., and achieve the effect of excellent gloss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0204] Hereinafter, the present invention will be described using examples, but the present invention is not limited to these examples. "%" means "wt%" unless otherwise specified.
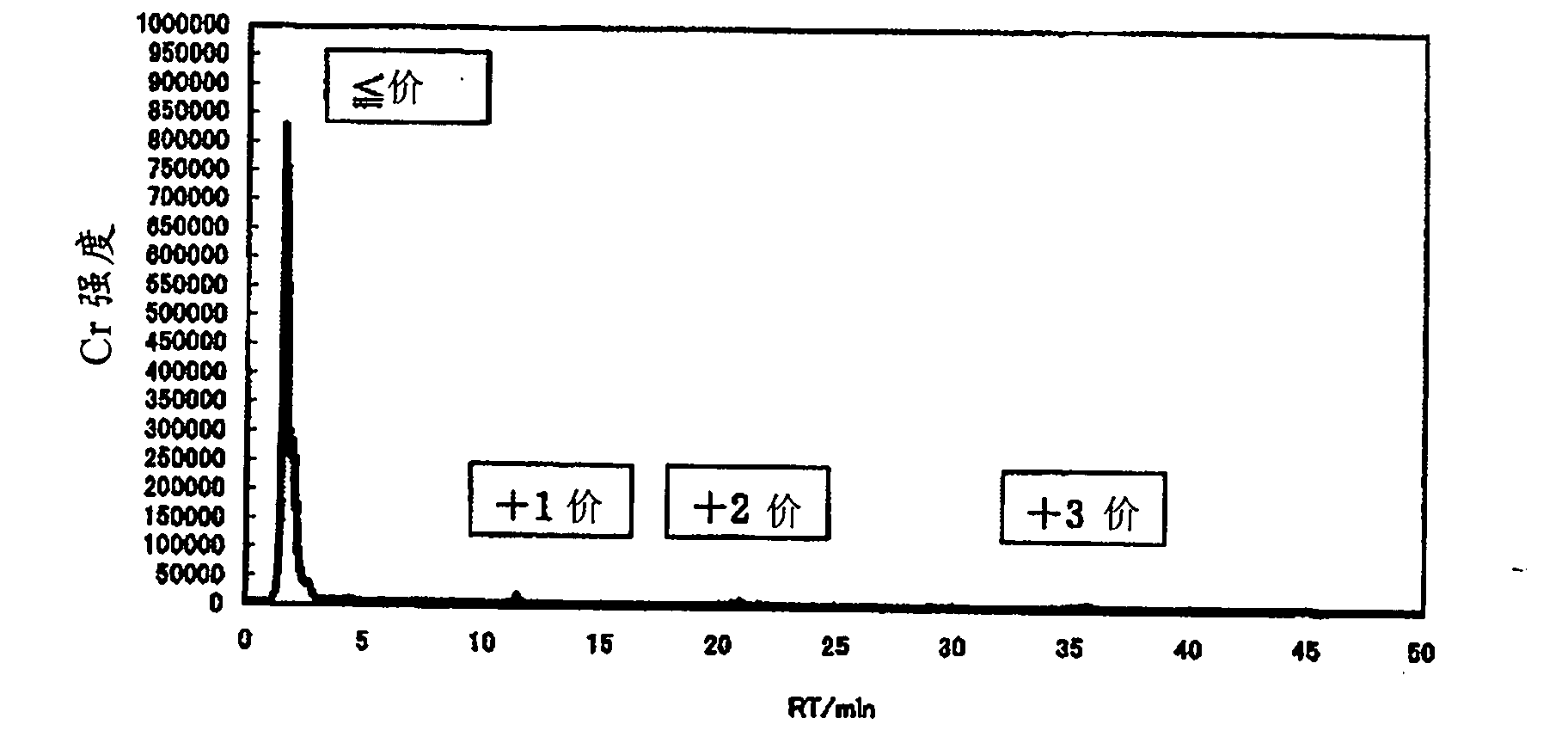
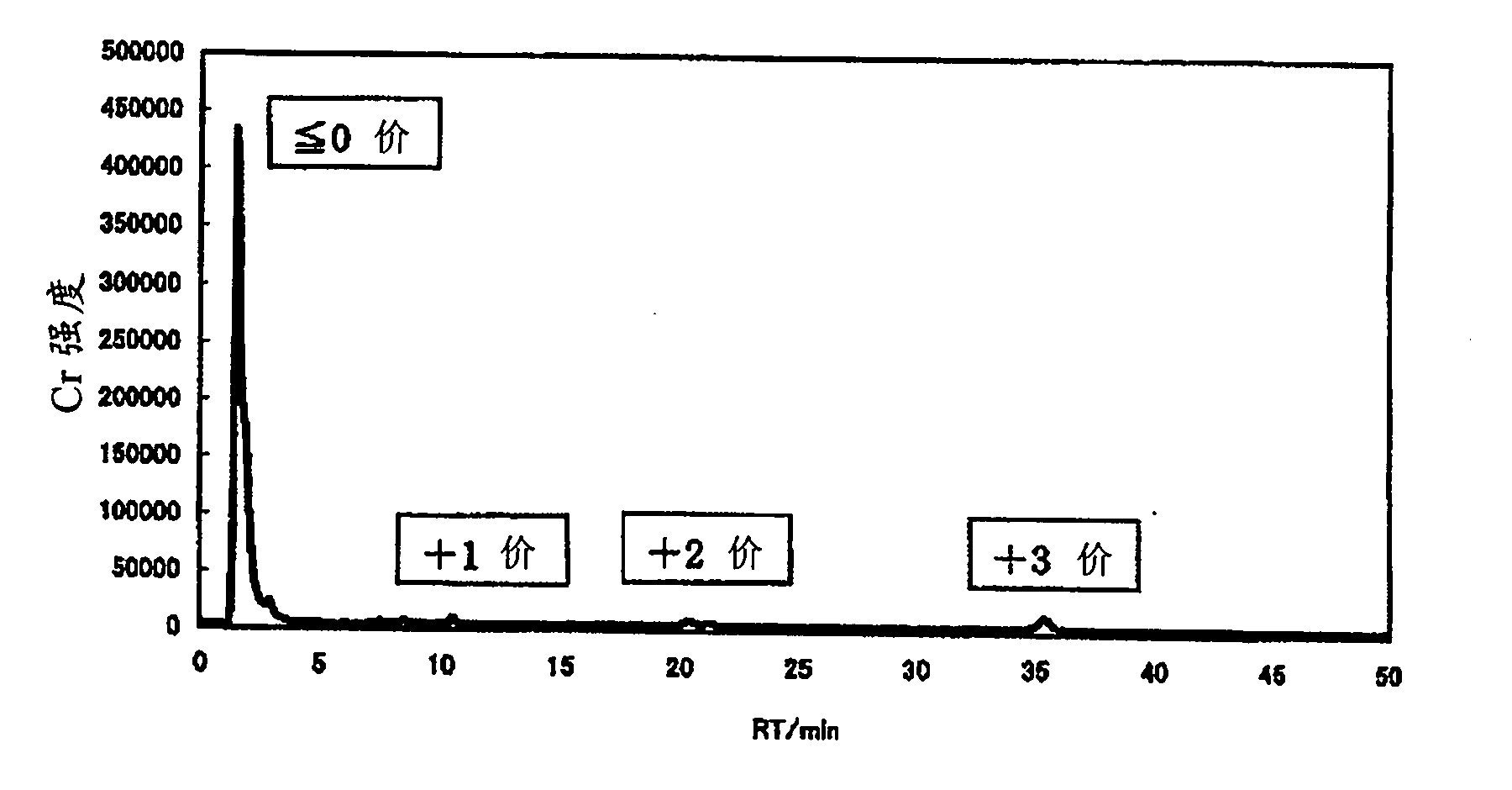
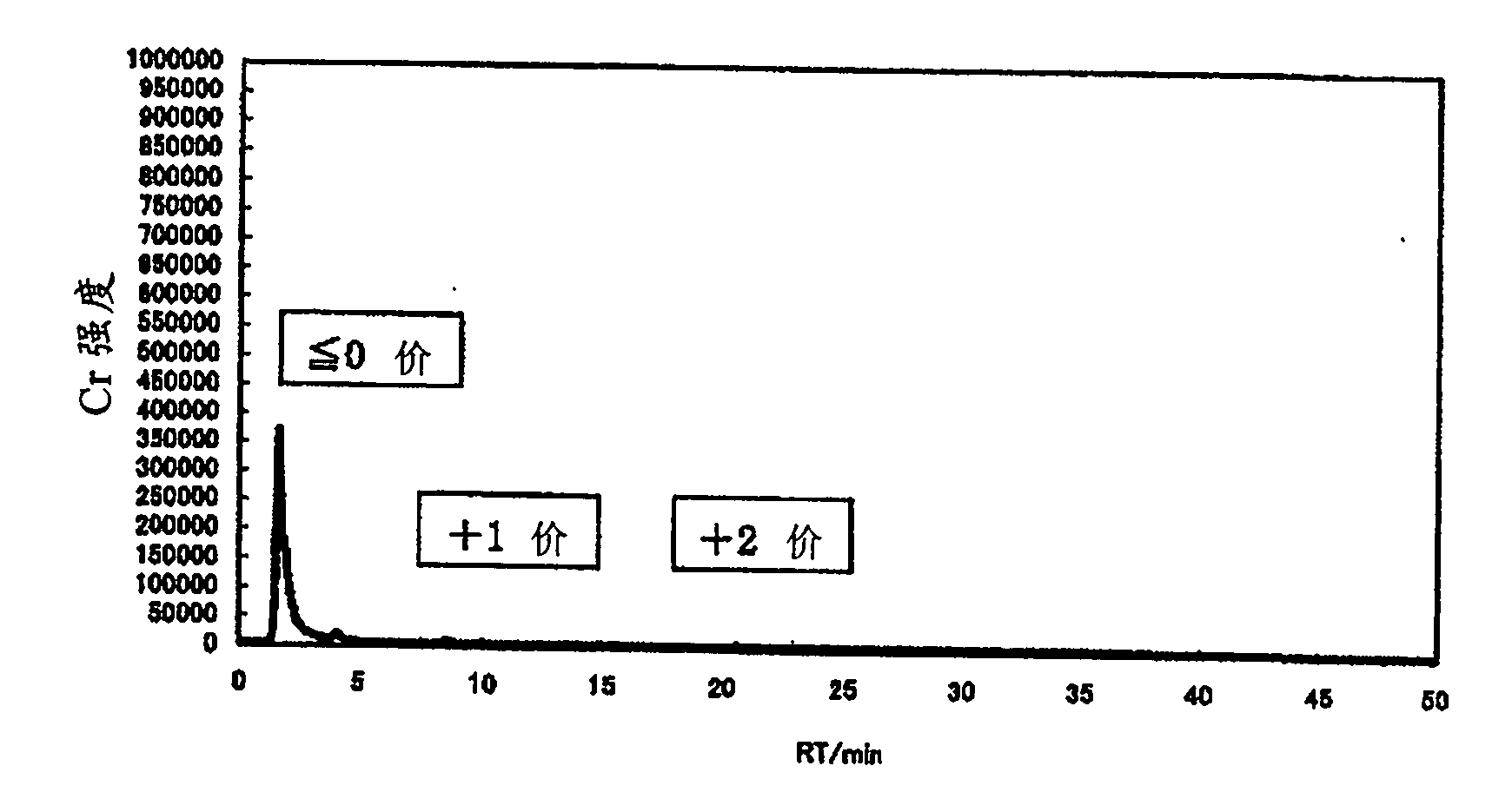
[0205] In an example, charge distribution of an aqueous solution containing chromium (III) was performed by diluting the aqueous chromium (III) solution with water to a total chromium concentration of 100 ppm using IC-ICP / AES (ion exchange separation-ICP emission analysis).
[0206] An ion-exchange separation device (DX-500, manufactured by DIONEX Corporation, Japan) was set to the following conditions.
[0207] Ion exchange resin: ethylvinylbenzene-divinylbenzene polymer (5.5 μm in diameter)
[0208] Eluent: methanesulfonic acid aqueous solution (the concentration of methanesulfonic acid increases linearly from 2mmol / L to 40mmol / L over time in 30 minutes, and is maintained at 40mmol / L after 30 minutes)
[0209] ·Flow rate: 1.2ml / min
[0210] ・Introduction volume of measurement object liquid: 20...
example 1
[0217] {instance 1}
[0218] (step 1)
[0219] 325.3 g of water was put into a glass-made reaction container with a condenser, and 124.9 g of chromium trioxide was further added, and fully stirred to obtain a chromic acid aqueous solution. Next, 292.8 g of 75% phosphoric acid was added to prepare a mixed aqueous solution of chromic acid and phosphoric acid.
[0220] Ethylene glycol is used as a reducing agent. 96.1 g of 20% ethylene glycol aqueous solution obtained by adding 76.6 g of water to 19.5 g of 98.5% ethylene glycol was added to the mixed aqueous solution of chromic acid and phosphoric acid at a rate of about 0.8 mL / min using a metering pump. The addition time was about 2 hours and the temperature was 102°C. 19.5 g of 98.5% ethylene glycol is equivalent to reducing 83% chromic acid.
[0221] (step 2)
[0222] Next, 216.2g of oxalic acid aqueous solution (containing 35% (COOH) 2 ), was added to the solution obtained in the first step at a rate of about 1.6mL / min. ...
example 2
[0224] {instance 2}
[0225] (step 1)
[0226] 512.9 g of water was added to a glass-made reaction vessel with a condenser, and 81.9 g of chromium trioxide was further added, and stirred well to obtain a chromic acid aqueous solution. Next, 106.7 g of 75% phosphoric acid was added to prepare a mixed aqueous solution of chromic acid and phosphoric acid.
[0227] Ethylene glycol is used as a reducing agent. 48.6 g of 20% ethylene glycol aqueous solution obtained by adding 38.7 g of water to 9.9 g of 98.5% ethylene glycol was added to the mixed aqueous solution of chromic acid and phosphoric acid at a rate of about 0.8 mL / min using a metering pump. The addition time was about 1 hour and the temperature was 100°C. 9.9 g of 98.5% ethylene glycol is equivalent to reducing 64% chromic acid.
[0228] (step 2)
[0229] Secondly, 302.4g oxalic acid aqueous solution (containing 35% (COOH) 2 ), was added to the solution obtained in the first step at a rate of about 2.3mL / min. The a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com