Thiazolidine neuraminidase inhibitor and application thereof
A technology of thiazolidine acids and compounds, which is applied in thiazolidine neuraminidase inhibitors and their application fields, can solve the urgent problems of research and development of neuraminidase inhibitors, and achieve a significant effect of inhibiting neuraminidase activity in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
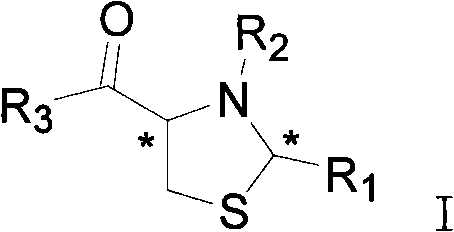
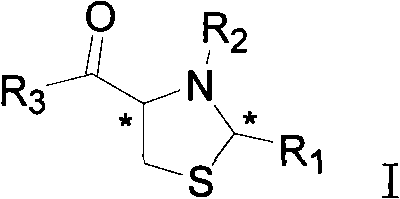
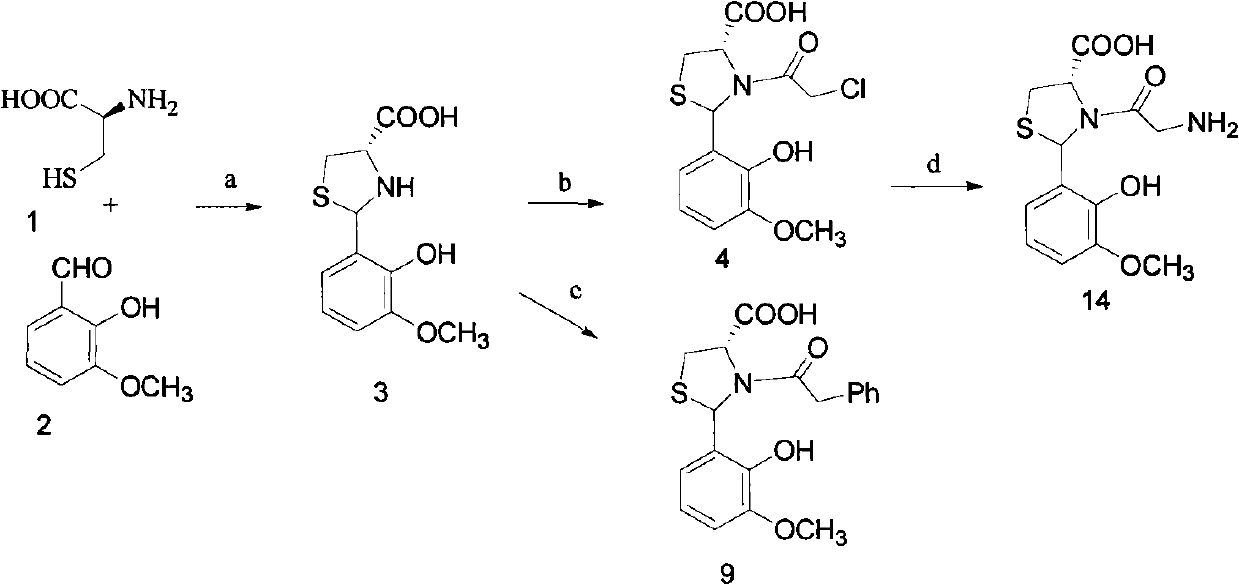
[0053] The preparation method of the compound of the above general formula I, the present invention uses cysteine 1 as a raw material, condenses with aldehyde 2 under alkaline conditions to form a thiazolidine intermediate 3, and then reacts with chloroacetyl chloride or phenylacetyl chloride in alkaline Condensation under the conditions to obtain the target compound (4) or (9), respectively; Compound (4) is further subjected to ammoniation, Boc protection and deprotection to obtain the target compound (14).
[0054] Taking o-vanillin (o-hydroxy-m-methoxybenzaldehyde) as an example below, the synthetic route and preparation method of this type of compound are illustrated.
[0055] synthetic route:
[0056]
[0057] Reagents: a. Sodium bicarbonate, ethanol / water; b. Chloroacetyl chloride, sodium bicarbonate / water; c. Phenylacetyl chloride, sodium bicarbonate / water; d. Ammonia; 2 O), sodium bicarbonate, tetrahydrofuran; ethyl acetate saturated with hydrogen chloride.
[0...
Embodiment 1
[0075] Example 1. Preparation of intermediate (2RS, 4R)-2-(2-hydroxyl-3-methoxyphenyl)-thiazolidine-4-carboxylic acid (1)
[0076] Dissolve 12.1g (0.11mol) of o-vanillin in 200mL of ethanol, and add 15.7g (0.1mol) of L-cysteine hydrochloride and 11.7g (0.11mol) of sodium bicarbonate into 200mL of aqueous solution under vigorous stirring . Stir at room temperature for 6h. Stand still, filter, the precipitate is washed with ethanol and water, and after drying, 23 g of the product is obtained, the yield is 92%, and the mp is 137-138°C. 1 H-NMR (DMSO-d 6 , 300MHz): 2.931-3.040(m, 1H, 5-H), 3.171-3.368(m, 1H, 5-H), 3.777-3.858(m, 3H, -OCH 3 ), 3.836(t, 0.46H, 4-H), 4.207(t, J=5.4Hz, 0.54H, 4-H), 5.671(s, 0.46H, 2-H), 5.865(s, 0.54H, 2 -H), 6.704-6.971 (m, 3H). HRMS: m / z 256.0643.
Embodiment 2
[0077] Example 2.3-Chloroacetyl-(2RS, 4R)-2-(2-hydroxy-3-methoxyphenyl)-thiazolidine-4-carboxylic acid (4)
[0078] Intermediate (2RS, 4R)-2-(2-hydroxyl-3-methoxyphenyl)-thiazolidine-4-carboxylic acid 2.6g (0.01mol) and sodium bicarbonate 2.1g (0.02 mmol) was dissolved in 100 mL of water, 0.9 mL (0.011 mmol) of chloroacetyl chloride was added dropwise under ice-bath conditions, and the reaction was stirred for 4 h. Use sodium bicarbonate to adjust the pH of the reaction solution to 8-9, wash with dichloromethane 3 times, each dosage 50mL (50mL×3), the aqueous phase is adjusted to pH 2-3 with citric acid, dichloromethane extracted 3 times each dosage 50mL (50mL×3), combined the organic phases, dried, evaporated to dryness, purified by column chromatography to obtain 2g of the final product, yield 60.4%, mp 130-132°C, 1 H-NMR (CDCl 3 , 300MHz): 3.325-3.464 (m, 2H, 5-H), 3.773-3.818 (d, 1H, J=13.5Hz, Cl-CH 2 -), 3.916(s, 3H, -OCH 3 ), 4.010-4.055(d, 1H, J=13.5Hz, Cl-CH2-), 5....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com