Copper complex of phenanthroline derivatives and preparation method and application thereof
A technology of o-phenanthroline and copper complexes, applied in the direction of copper organic compounds, drug combinations, 1/11 group organic compounds without C-metal bonds, etc., to achieve high yield, good water solubility and fat solubility, excellent The effect of inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of complex Z1
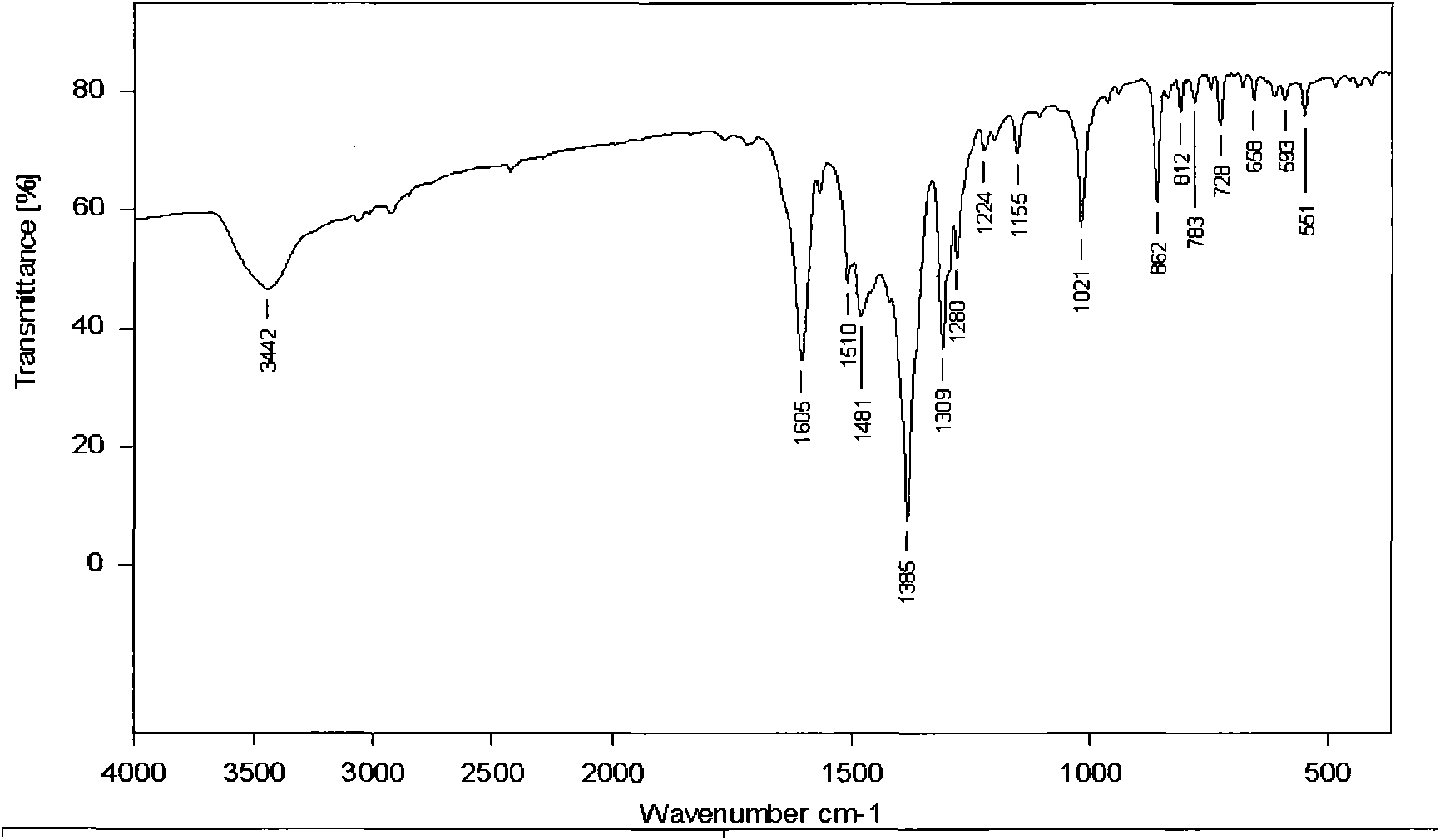
[0038] 0.29g Cu(NO 3 ) 2 ·3H 2 O and 0.174g of 2,9-dimethyl-1,10-phenanthroline were sequentially added into 10mN,N-dimethylacetamide (DMA) and 50ml of anhydrous methanol, stirring continuously. After reacting for 8 hours, it was filtered, the mother liquor was diffused with diethyl ether, and allowed to stand at room temperature, and green blocky crystal Z1 was obtained after a few days.
[0039] The molecular formula of Z1 compound is: {Cu[2,9-CH 3 -(1,10-Phen)](NO 3 ) 2 (DMA)}, i.e. 1 2,9-CH 3 -(1,10-Phen), 2 NO 3 and 1 DMA coordinated to 1 Cu.
[0040] Elemental analysis (%): Calculated (exp.): C 44.77 (44.66); H 4.35 (3.30); N 14.51 (14.46); Cu 13.16 (13.19).
[0041] Its infrared spectrum and X-ray diffraction single crystal structure diagram are as follows Figure 1A and Figure 1B shown.
[0042] Cell experiment of complex Z1
[0043] Adherent tumor cells in the logarithmic growth phase were selected: A-549 (lung cancer),...
Embodiment 2
[0056] Preparation of Complex Z2
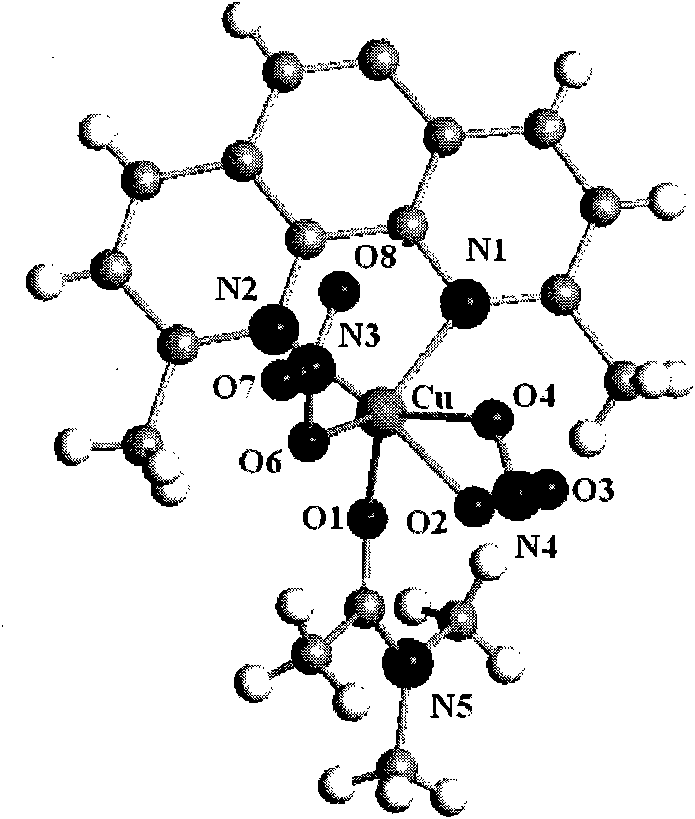
[0057] 0.29g Cu(NO 3 ) 2 ·3H 2 O and 0.174g of 2,9-dimethyl-1,10-phenanthroline were sequentially added into 50ml of N,N-dimethylformamide (DMF) and stirred continuously. After reacting for 8 hours, it was filtered, the mother liquor was diffused with diethyl ether, and stood at room temperature. After several days, green blocky crystal Z2 was obtained.
[0058] The molecular formula of the Z2 compound is: {Cu[2,9-CH 3 -(1,10-Phen)](NO 3 ) 2 (DMF)}, i.e. 1 2,9-CH 3 -(1,10-Phen), 2 NO 3 And 1 DMF is coordinated with 1 Cu.
[0059] Elemental analysis (%): Calculated (exp.): C 43.54 (40.85); H 4.06 (3.36); N 19.94 (19.88); Cu 13.55 (13.87).
[0060] Its infrared spectrum and X-diffraction single crystal structure diagram are as follows Figure 2A and Figure 2B shown.
[0061] Cell experiment of complex Z2
[0062] Adherent tumor cells in the logarithmic growth phase were selected: A-549 (lung cancer), Bel-7402 (liver cancer), HCT (...
Embodiment 3
[0075] Preparation of Complex Z3
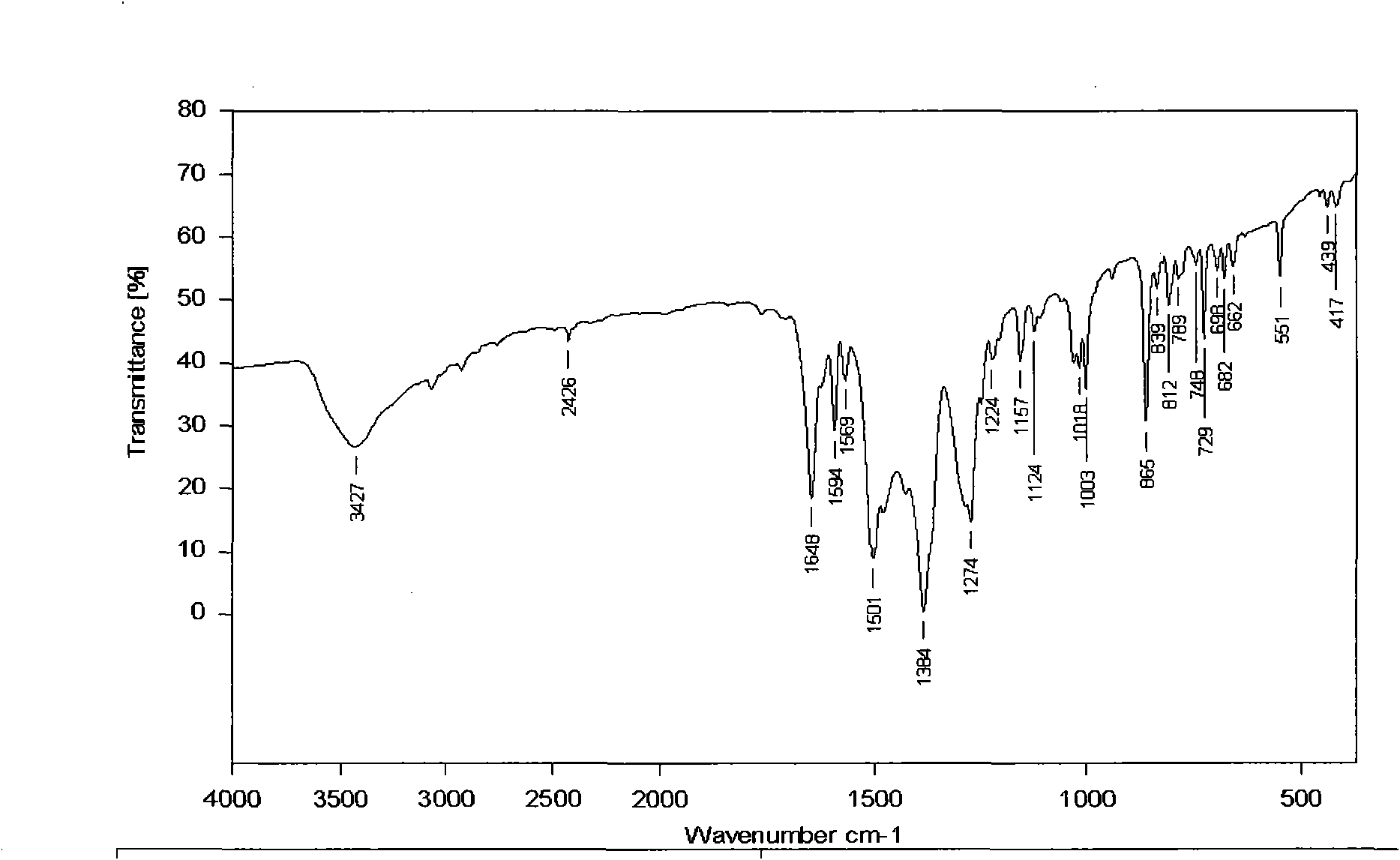
[0076] 0.29g Cu(NO 3 ) 2 ·3H 2 O and 0.174g of 2,9-dimethyl-1,10-phenanthroline were sequentially added to a mixed solution of 25mL of methanol and 25mL of acetonitrile, and the mixture was continuously stirred. After reacting for 8 hours, it was filtered, the mother liquor was diffused with diethyl ether, and allowed to stand at room temperature. After a few days, green blocky crystal Z3 was obtained.
[0077] The molecular formula of Z3 compound is: {Cu[2,9-CH 3 -(1,10-Phen)](NO 3 ) 2 (CH 3 OH)}, namely 1 2,9-CH 3 -(1,10-Phen), 2 NO 3 and 1 CH 3 OH coordinates with 1 Cu.
[0078] Elemental analysis: Calculated (exp.): C 42.11 (40.85); H 3.74 (3.36); N 13.10 (13.88); Cu 14.85 (14.67).
[0079] Its infrared spectrum and X-diffraction single crystal structure diagram are as follows Figure 3A and Figure 3B shown.
[0080] Cell experiment of complex Z3
[0081]Adherent tumor cells in the logarithmic growth phase were selected: A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com