Method for synthesizing 16 alpha-hydroxy prednisolone
A technology of hydroxyprednisolone and synthetic methods, applied in the direction of steroids, organic chemistry, etc., can solve the problems of key technologies that need to be broken through and the inability to form industrial production, and achieve the effects of low difficulty, easy control, and low pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
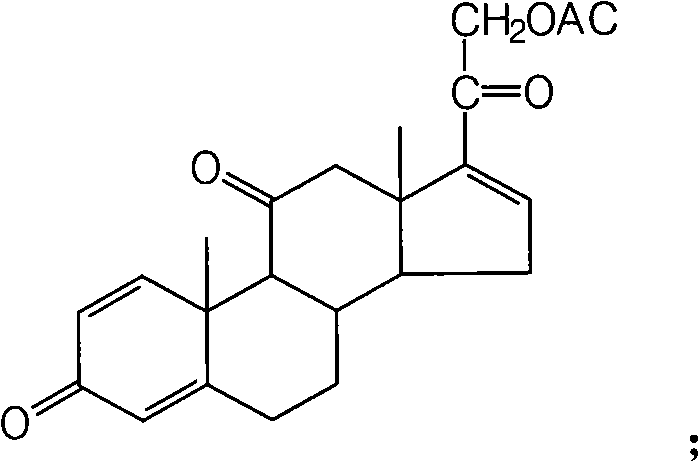
[0030] This example introduces a synthetic method for 16α-hydroxyprednisolone. The synthetic steps are as follows: firstly add 40g of prednisone, 240ml of pyridine, and 100ml of acetone into the reaction bottle in turn, and keep the reaction below 0°C to achieve Detected under 254nm ultraviolet light, after the raw material point is less than 1%, water analysis in ice water, standing overnight, suction filtration, washing with water, drying, recrystallization to obtain 31.2g of intermediate I, the chemical formula of intermediate I is:
[0031]
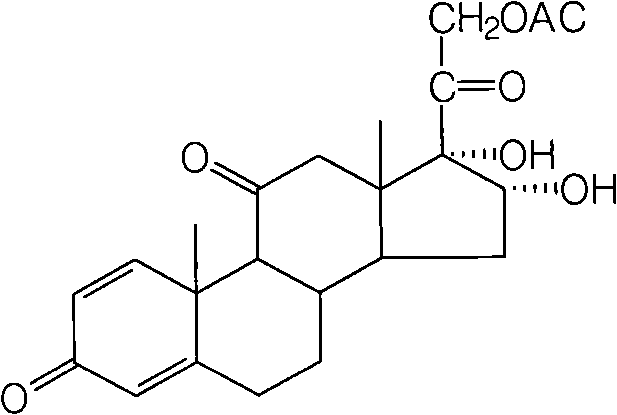
[0032] Take 20g of intermediate I, 11g of potassium permanganate, 2ml of triethylamine, and 1000ml of acetone, and put them into the reaction bottle in turn, react at low temperature, and achieve detection under a 254nm ultraviolet lamp. After the raw material point is less than 1%, add sulfite 5g, terminate the reaction, filter, concentrate under reduced pressure, cool, suction filter, and dry to obtain 20g of intermediate II, the ...
Embodiment 2
[0041] This example introduces a synthesis method of 16α-hydroxyprednisolone. The synthesis steps are as follows: first, add 40 g of prednisone, 240 ml of collidine, and 100 ml of acetone into the reaction bottle in sequence, and keep the temperature below 0°C for reaction , to achieve detection under 254nm ultraviolet light, after the raw material point is less than 1%, water analysis in ice water, standing overnight, suction filtration, washing with water, drying, recrystallization to obtain 31.2g of intermediate I, the chemical formula of intermediate I is:
[0042]
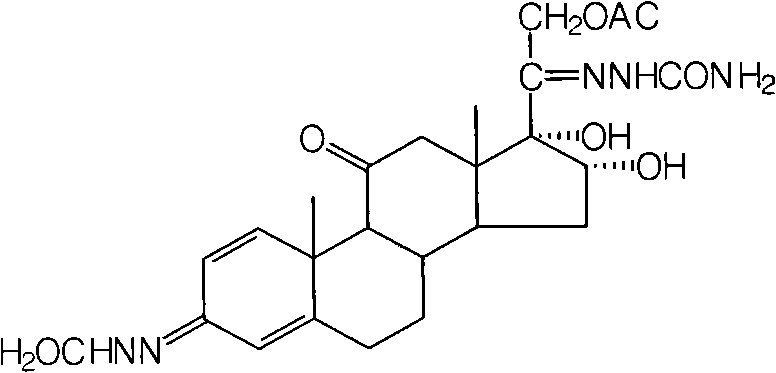
[0043] Take 20g of intermediate I, 11g of osmium tetroxide, 2ml of pyridine, and 1000ml of acetone and put them into the reaction bottle in turn, and react at low temperature until it is detected under a 254nm ultraviolet lamp. After the raw material point is less than 1%, add 5g of sulfite to stop Reaction, filtration, concentration under reduced pressure, cooling, suction filtration, drying to obtain 20g o...
Embodiment 3
[0052] This example introduces a synthetic method for 16α-hydroxyprednisolone. The synthetic steps are as follows: first, add 40 g of prednisone, 120 ml of pyridine, 120 ml of collidine, and 100 ml of acetone into the reaction flask in sequence, The following heat preservation reaction can be detected under a 254nm ultraviolet lamp. After the raw material point is less than 1%, the ice water water analysis, standing overnight, suction filtration, washing with water, drying, recrystallization to obtain 31.2g of intermediate I, the chemical formula of intermediate I for:
[0053]
[0054] Take 20g of intermediate I, 5g of potassium permanganate, 5g of osmium tetroxide, 2ml of collidine, and 1000ml of acetone and put them into the reaction bottle in turn, and react at low temperature to achieve detection under 254nm ultraviolet lamp, and the raw material point is less than 1%. Finally, add sulfite 5g, stop reaction, filter, concentrate under reduced pressure, cool, suction fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com