Kit for determining small-particle size homogeneous sol particle type cystatin C and preparation method therefor
A kit, particle type technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] A kit for measuring cystatin C in homogeneous sol particle type with small particle size and a preparation method thereof.
[0015] 1. The preparation of reagent R1 of the present invention
[0016] Prepare according to conventional methods.
[0017] 2. The preparation of reagent R2 of the present invention
[0018] I. Preparation of Colloidal Gold Solution
[0019] (1) prepare 1% chloroauric acid and 10% trisodium citrate with ultrapure water;
[0020] (2) Ultrapure water prepares 500ml of tetrachloroauric acid per ten thousandths (20ml 1% chloroauric acid adds 480ml ultrapure water);
[0021] (3) Heat and stir until slightly boiled, and quickly add trisodium citrate (i.e. 4ml 10% trisodium citrate) with a mass ratio of 1.2:1 to chloroauric acid;
[0022] (5) Heat and stir until the color turns into wine red, continue heating and stirring for 5-8 minutes and stop;
[0023] (6) Use an ultraviolet spectrophotometer to detect the absorbance value at 500nm-560nm, and ...
Embodiment 2
[0031] Embodiment 2 working curve is measured
[0032] The dosage of the present invention: the dosage of the reagent R1 of the present invention and the reagent R2 of the present invention are 160ul and 40ul respectively, and the dosage of the sample is 2ul.
[0033] The present invention adopts the endpoint method for measurement: 160ul R1 is added to 2ul sample, 40ul reagent R2 is added after 5 minutes at 37°C, and the reading point is started, and the point is read again after 5 minutes of reaction to obtain the absorbance difference.
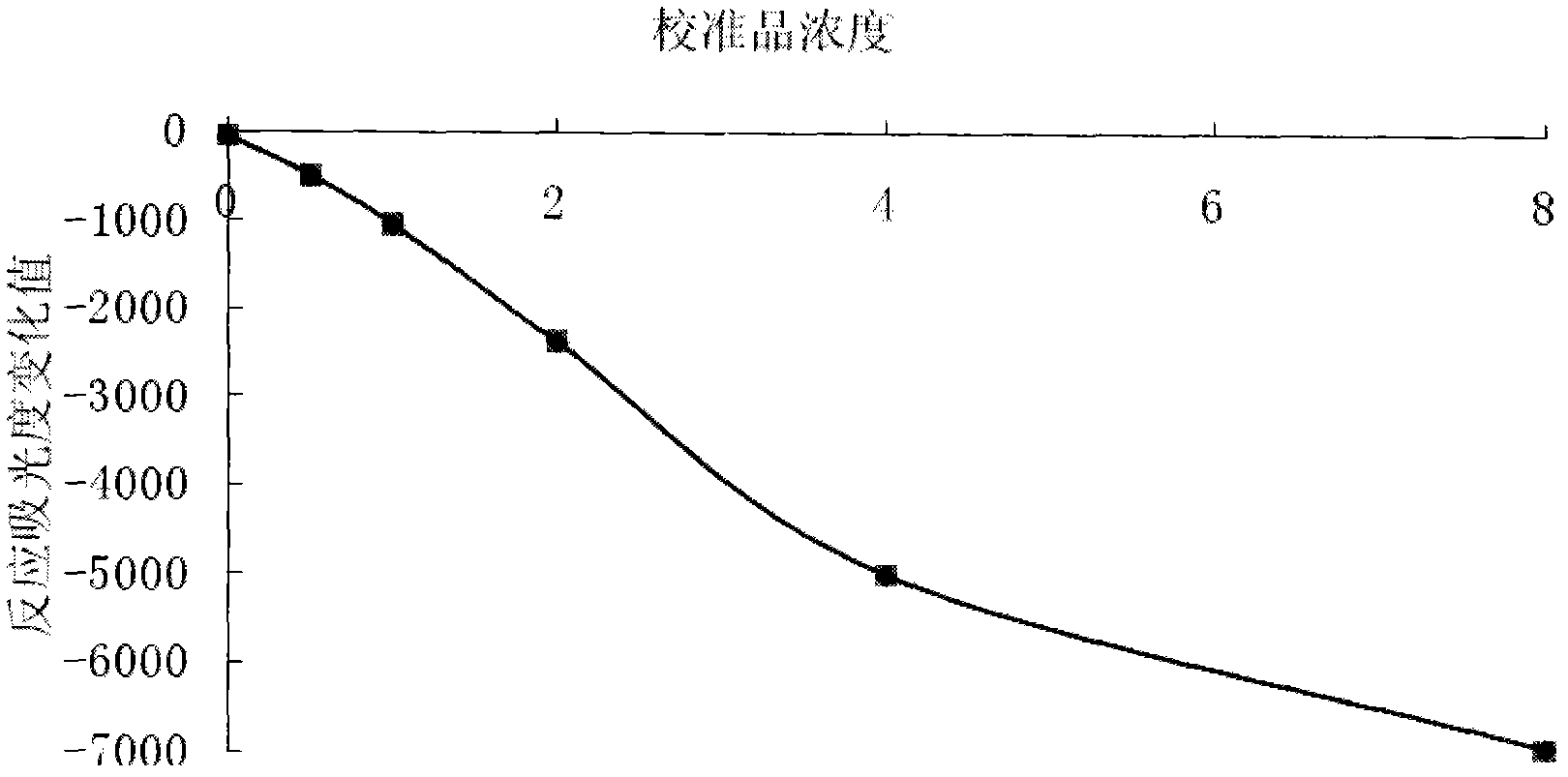
[0034] Make the standard curve of the present invention: adopt the standard product of the present invention (choose 6 points to calibrate, calibrator cystatin C content is respectively 0.0, 0.5, 1.0, 2.0, 4.0, 8.0 mg / ml), use Mindray BS300 automatic biochemical instrument, the main detection wavelength is 546nm, and the secondary wavelength is 660nm;
[0035] According to the above-mentioned assay steps, the standard curve (such as figur...
Embodiment 3
[0036] Embodiment 3 accuracy, precision measurement
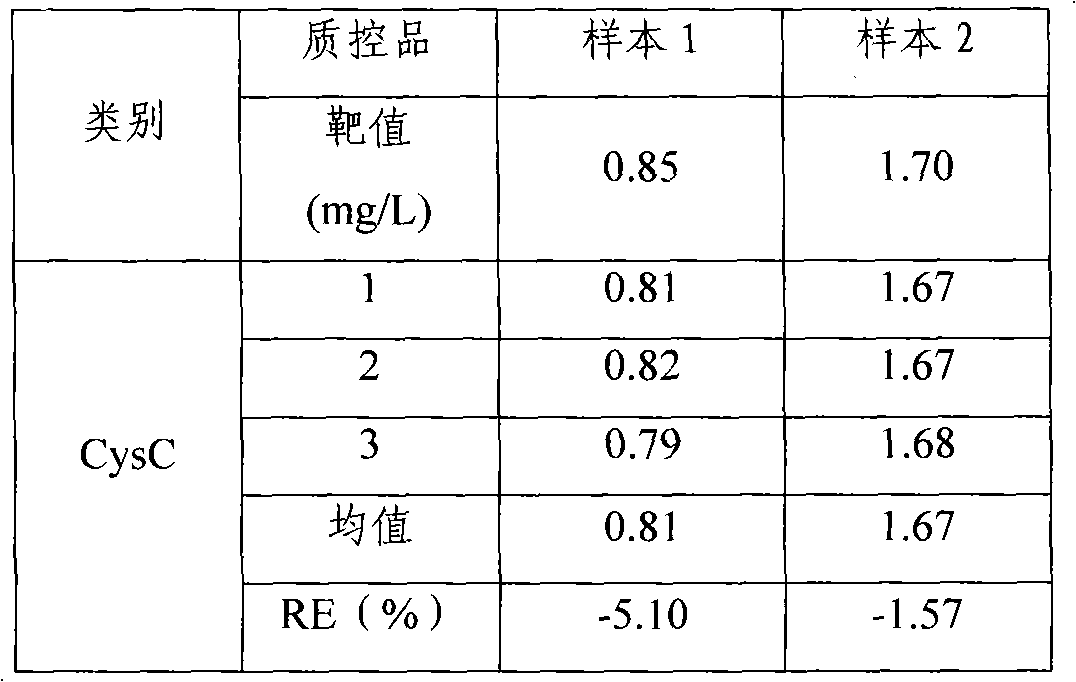
[0037] Utilize the quality control product provided by China Jiuqiang Company, choose two concentrations (lower and higher concentration), measure with the test kit of the present invention, the result is as follows.
[0038] Table 1
[0039]
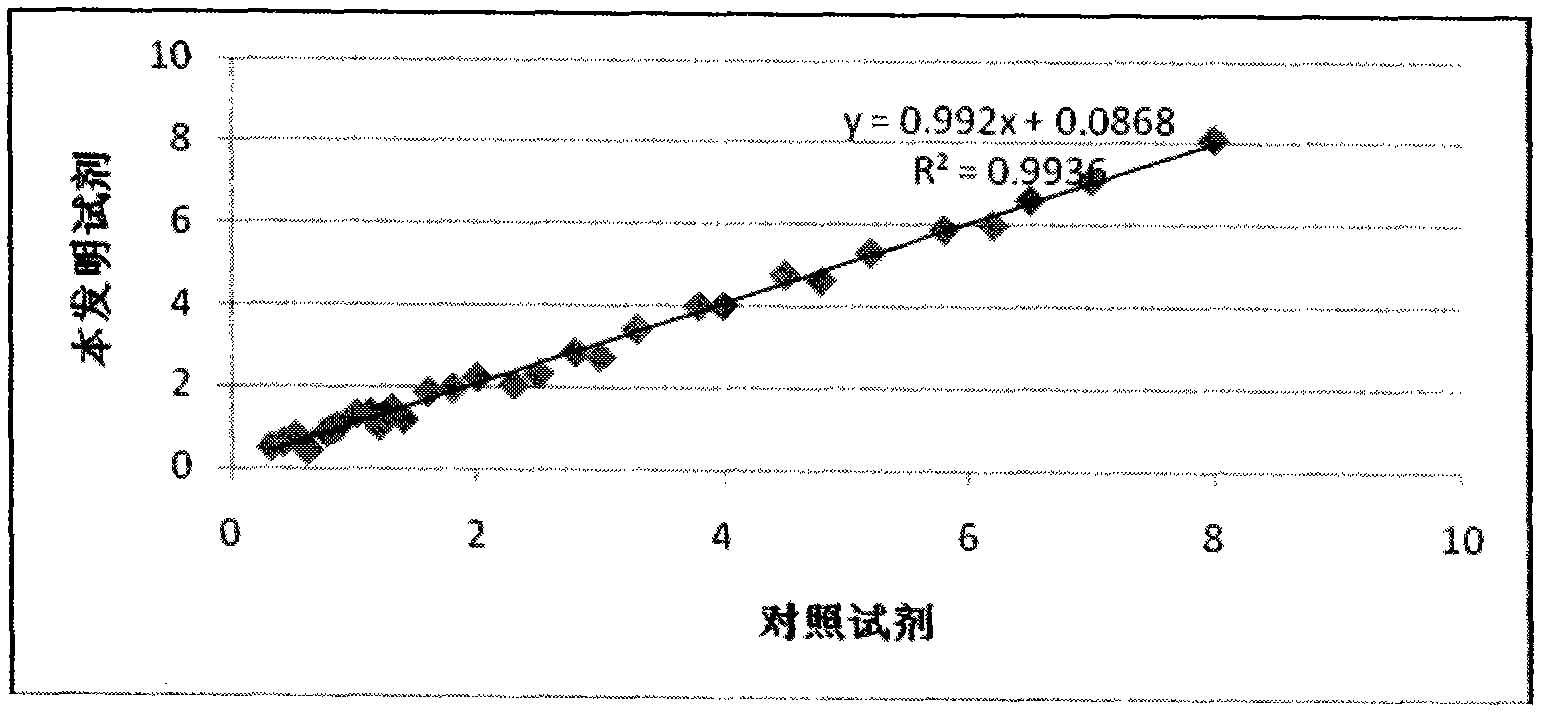
[0040] Correlation experiment: using the reagent of the present invention and a control reagent (cystatin C kit of a well-known international company). The mindray BS300 automatic biochemical analyzer was used to measure 30 human sera, and the correlation analysis was carried out on the measured values. See the test results figure 2 , the X and Y axes in the figure are measured values (cystatin C content mg / L).
[0041] Measurement of precision: Randomly select a normal serum sample and a serum sample of a patient with kidney disease for 20 consecutive measurements, and calculate according to CV(%)=SD / Mean×100.
[0042] Table 2
[0043] determination
[0044] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com