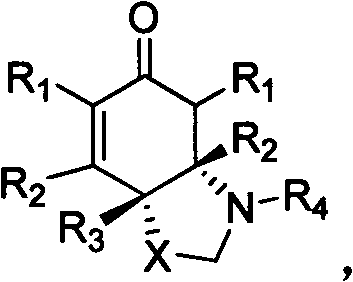
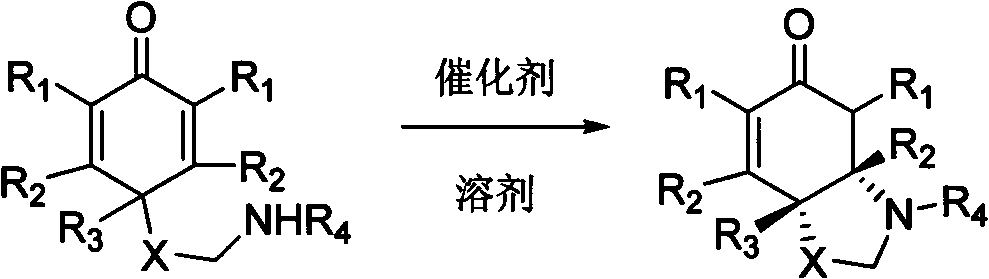
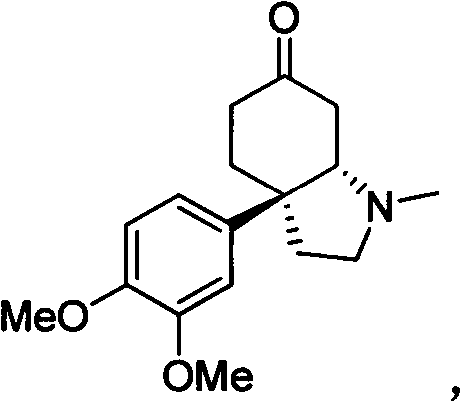
Chiral nitrogen-containing heterocyclic compound, and synthesis method and application thereof
A nitrogen heterocyclic compound and compound technology, applied in the fields of chiral nitrogen-containing heterocyclic compound, synthesis and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the preparation of chiral phosphoric acid
[0019] Under the protection of argon at room temperature, dissolve the derivative of BINOL (0.5mmol) in 1mL of dry pyridine in a dry reaction tube, and slowly drop (1.0mmol) of phosphine oxychloride under the condition of rapid stirring Added to the system, stirred at room temperature for 3 hours. 1 mL of water was slowly added dropwise to the system, and stirred at room temperature for 30 minutes. Dichloromethane was added to dissolve, washed with 1N aqueous hydrochloric acid (3×10 mL), the organic layer was dried over anhydrous sodium sulfate, the solvent was spun off under reduced pressure, and the residue was separated by column chromatography to obtain the product.
[0020] (S)-3,3'-[3,5-bis(trifluoromethyl)phenyl]2-1,1'-binaphthol phosphate (1a)
[0021] (S)-3,3′-[3,5-Bis(trifluoromethyl)phenyl]2-1,1′-binaphthyl phosphate(1a)
[0022]
[0023] White solid, 89% yield. 1 H NMR (400MHz, CDCl 3 )δ8.01(s...
Embodiment 2
[0024] Embodiment 2: the preparation of chiral thiourea
[0025] Under the protection of argon at room temperature, 9-amino cinchonine (2.93g, 10mmol) was dissolved in dry tetrahydrofuran (30mL) in a dry reaction tube, and 3,5-bistrifluoromethylbenzene isothiocyanate was added at room temperature A solution of the acid ester (2.70 g, 10 mmol) in tetrahydrofuran (10 mL). After reacting overnight, the solvent was spinned off under pressure, and the residue was separated by column chromatography (EtOAc / MeOH / =300 / 5) to obtain the catalyst thiourea.
[0026] 1-(3,5-bistrifluoromethylphenyl)3-(R)-4-quinoline-(2R,4S,5R)-5-vinyl-2-quininemethylthiourea (2a) 1-(3,5-bis(trifluoromethyl)phenyl)-3-((R)-quinolin-4-yl((2R,4S,5R)-5-vinylquinuclidin-2-yl)methyl)thiourea(2a)
[0027]
[0028] White solid, 80% yield. 1 H NMR (300MHz, CD 3 OD) δ0.77-0.85(m, 2H), 1.12-1.20(m, 1H), 1.43-1.51(m, 3H), 2.25-2.28(m, 1H), 2.89-2.99(m, 3H), 3.11 -3.24(m, 3H), 5.08-5.14(m, 2H), 5.80-5.92(m, 1H), ...
Embodiment 3
[0029] Example 3: Intramolecular Aza-Michael Reaction Catalyzed by Chiral Thiourea
[0030]
[0031] Method A: Under argon protection, add cyclohexadienone derivative (0.3 mmol), chiral thiourea catalyst 2a (8.5 mg, 5 mol%) and dichloromethane (3 mL) in a dry reaction tube. React at room temperature until the starting material disappears (TLC detection). The solvent was spun off under reduced pressure, and the residue was separated by plate chromatography to obtain the product.
[0032] Method B: Under argon protection, add cyclohexadienone derivative (0.3mmol), chiral thiourea catalyst 2a (33.8mg, 20mol%), and dichloromethane (0.6mL) in a dry reaction tube . The reaction was heated to reflux until the starting material disappeared (TLC detection). The solvent was spun off under reduced pressure, and the residue was separated by plate chromatography to obtain the product.
[0033]
[0034] (3aS,7aS)-3a-Hydroxy-1-p-methylbenzenesulfonyl-3,3a,7,7a-tetrahydro-1H-indol-6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com