Flexible multi-arm porphyrin with pyridine groups and synthesis method thereof
A pyridine group, synthesis method technology, applied in flexible multi-arm porphyrin and its synthesis, flexible multi-arm porphyrin with pyridine group and its synthesis field, to achieve convenient operation, high yield and good coordination ability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
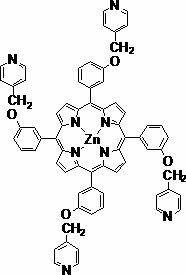
[0036] (1) Synthesis of 5,10,15,20-tetrakis(3-hydroxyphenyl)porphyrin
[0037] Add 9.1637g (75mmol) of m-hydroxybenzaldehyde to 100ml of propionic acid. After the temperature of the system reaches 130°C, add 5.3ml (75mmol) of pyrrole, reflux for 1h under nitrogen protection, cool to room temperature, stir for 24h, and filter. Rinse with hot distilled water at 80°C, dry in the air overnight, and separate by column chromatography (silica gel as stationary phase, chloroform / methanol (volume ratio: 9:1) as eluent), collect 5,10, 15,20-Tetrakis(3-hydroxyphenyl)porphyrin, 10% yield.
[0038] (2) Synthesis of 5,10,15,20-tetrakis(3-hydroxyphenylzinc)porphyrin
[0039] Add 0.4g (0.059mmol) of 5,10,15,20-tetrakis(3-hydroxyphenyl)porphyrin and 0.9g (0.47mmol) of zinc acetate to the mixed solution of chloroform and methanol (the volume ratio of the two is 1:1), reacted at 65°C for 3 hours, after the reaction was completed, the solvent was removed, and the crude product was separated by ...
Embodiment 2
[0044](1) Synthesis of 5,10,15,20-tetrakis(3-hydroxyphenyl)porphyrin
[0045] Add 9.1637g (75mmol) of m-hydroxybenzaldehyde to 100ml of propionic acid. After the temperature of the system reaches 140°C, add 5.3ml (75mmol) of pyrrole, reflux for 2h under the protection of nitrogen, cool to room temperature, stir for 20h, and filter. Rinse with hot distilled water at 80°C, dry in the air overnight, separate by column chromatography (silica gel as stationary phase, use chloroform / methanol (volume ratio 9:1) as eluent), collect 5,10,15 , 20-tetrakis (3-hydroxyphenyl) porphyrin, yield 6.5%.
[0046] (2) Synthesis of 5,10,15,20-tetrakis(3-hydroxyphenylzinc)porphyrin
[0047] Add 0.4g (0.059mmol) of 5,10,15,20-tetrakis(3-hydroxyphenyl)porphyrin and 0.9g (0.47mmol) of zinc acetate to the mixed solution of chloroform and methanol (the volume ratio of the two is 1:1.5), reacted at 70°C for 4 hours, after the reaction was completed, the solvent was removed, and the crude product was se...
Embodiment 3
[0051] (1) Synthesis of 5,10,15,20-tetrakis(3-hydroxyphenyl)porphyrin
[0052] Add 9.1637g (75mmol) of m-hydroxybenzaldehyde to 100ml of propionic acid. After the temperature of the system reaches 130°C, add 8ml (112.5mmol) of pyrrole, reflux for 1h under nitrogen protection, cool to room temperature, stir for 24h, and filter. Rinse with hot distilled water at 80°C, dry in the air overnight, separate by column chromatography (silica gel as stationary phase, use chloroform / methanol (volume ratio 9:1) as eluent), collect 5,10,15 ,20-Tetrakis(3-hydroxyphenyl)porphyrin, yield 7%.
[0053] (2) Synthesis of 5,10,15,20-tetrakis(3-hydroxyphenylzinc)porphyrin
[0054] Add 0.4g (0.059mmol) of 5,10,15,20-tetrakis(3-hydroxyphenyl)porphyrin and 0.45g (0.24mmol) of zinc acetate into the mixed solution of chloroform and methanol (the volume ratio of the two is 1:2), reacted at 70°C for 4 hours, after the reaction was completed, the solvent was removed, and the crude product was separated b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com