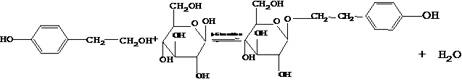
Method for synthesizing salidroside by utilizing enzyme catalyzed direct glucosylation
A technology of salidroside and chemical synthesis, applied in chemical recovery, fermentation, etc., to achieve the effects of reducing water activity, improving solubility, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 55 μL of citric acid-phosphate buffer solution, 396 μL 1,4-dioxane and 99 μL 1-butyl-3-methylimidazolium hexafluorophosphate in a 10 ml test tube, add 22 mg glucose, 33 mg tyrosol to the reaction medium , adding 1.1 mg of enzyme to initiate the reaction, shaking the reaction on a rotary shaker at 200 rpm for 2 days.
[0034] Sampling 550μL at the scheduled time, adding 8.5ml of methanol to terminate the reaction and ultrasonically oscillating for 20min, centrifuging at 14000rpm for 5min, filtering the supernatant with a 0.45um filter membrane, and using high performance liquid chromatography (LC-10A) to determine the yield of salidroside To measure. SPD-10A UV-visible detector; Elite C18 (4.6mm×250mm×5um); mobile phase methanol:water (15:85), flow rate 1ml / min; detection wavelength 275nm; column temperature room temperature; injection volume 20μL. The salidroside concentration in the reaction system was detected to be 3.6g / L.
Embodiment 2
[0036] Add 55 μL of citric acid-phosphate buffer solution, 500 μL of 1-hexyl-3-methylimidazolium hexafluorophosphate in a 10 ml test tube, add 22 mg of glucose, 33 mg of tyrosol, and 1.1 mg of enzyme to initiate the reaction at 200 rpm Shake the reaction on a rotary shaker for 2 days. The product salidroside was detected by the same detection method as in Example 1, and the product concentration could reach 0.58 g / L.
Embodiment 3
[0038] Add 55 μL of citric acid-phosphate buffer solution, 396 μL 1,4-dioxane and 99 μL 1-butyl-3-methylimidazolium hexafluorophosphate in a 10 ml test tube, add 22 mg glucose, 99 mg tyrosol to the reaction medium , adding 1.1 mg of enzyme to initiate the reaction, shaking the reaction on a rotary shaker at 200 rpm for 2 days. The product salidroside was detected by the same detection method as in Example 1, and the product concentration could reach 7.9 g / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com