Cholesterol modified anti-human immunodeficiency virus (HIV) polypeptide medicament and application thereof
A technology of cholesterol and modifiers, which is applied in the field of cholesterol-modified anti-HIV polypeptide drugs and their applications, which can solve the problems of easy drug resistance, limited application, and difficulty in developing into drugs due to water solubility, and achieve good water solubility and long half-life , easy to synthesize effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
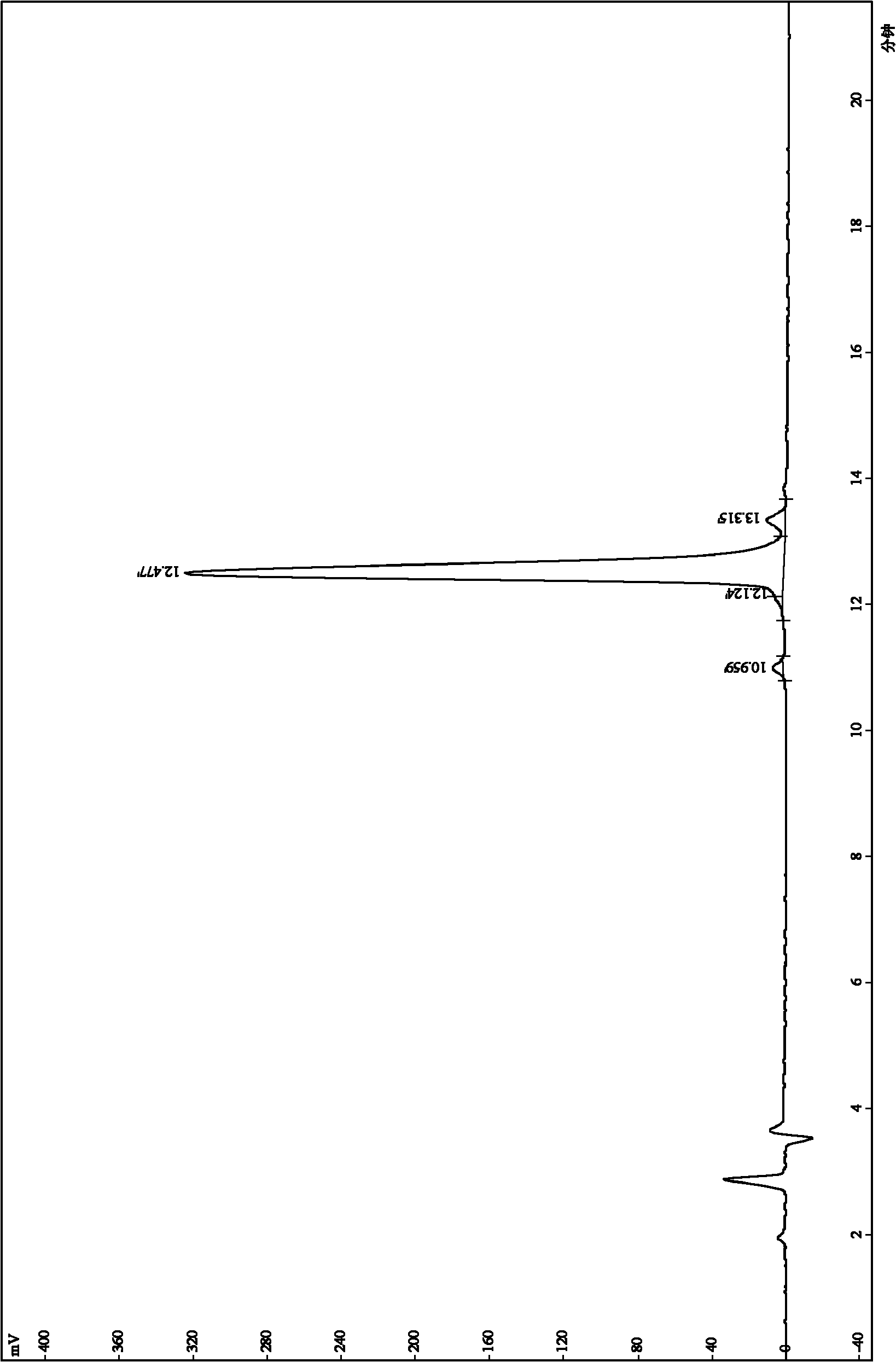
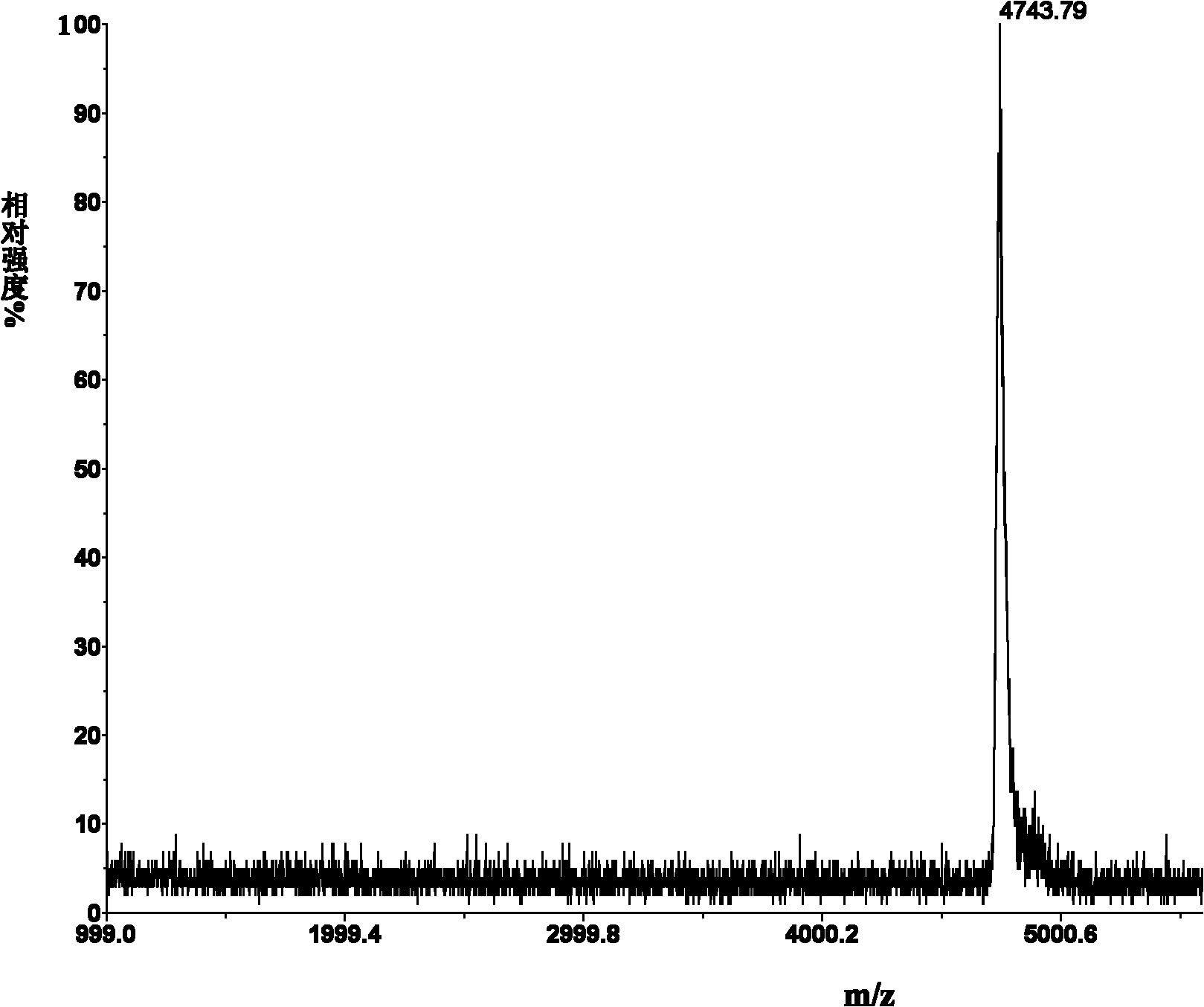
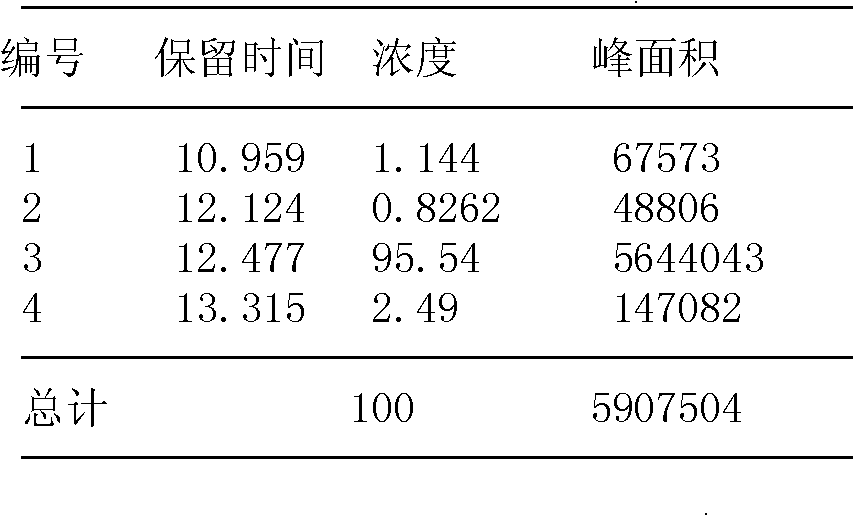
[0048] Example 1. Synthesis and modification of peptides
[0049] 1.1 Materials
[0050] The required chemical reagents were all obtained from major chemical reagent suppliers and used without further purification. Rink resin (with a substitution constant of 0.44 mmol / g) was purchased from Tianjin Hecheng Company.
[0051] 1.2 Polypeptide synthesis
[0052] All peptides were synthesized by solid-phase peptide synthesis (Fmoc / tBu strategy), manually synthesized from the carboxy-terminus to the amino-terminus.
[0053] Amino acid protected by N-fluorenylmethoxycarbonyl (Fmoc), Rink resin (substitution constant is 0.44mmol / g) is used as a solid phase carrier, and the amino protecting group is removed with a DMF solution of 25% (volume percentage) piperidine For Fmoc, each removal step needs to be carried out twice, and the duration is 8min and 10min respectively. The condensation methods used for the peptide grafting reaction were DIPC / HOBt method and PyBOP method. The amin...
Embodiment 2
[0068] Example 2. Evaluation of antiviral effect of CHFI polypeptide
[0069] 2.1 Inhibitory effect of polypeptide on HIV strain NL4-3: The molecular cloning plasmid pNL4-3 encoding HIV-1 strain NL4-3 was provided by NIH AIDS Research & Reference Reagent Program (catalogue number: 114). The pNL4-3 plasmid was prepared using the plasmid extraction kit from QIAGEN, and Lipofectamine from Invitrogen TM 2000 Transfection Reagent Transfect the pNL4-3 plasmid into 293T cells at 37°C in 5% CO 2 After 6 hours of incubation in the cell culture incubator, the medium was changed, and then culture was continued for 48 hours. Gently collect the supernatant in the cell culture flask or cell culture plate with a pipette, filter the supernatant through a 0.45 μm filter, add 20% fetal bovine serum (FBS), then divide into polypropylene tubes, and place at -80 Store at ℃ for later use or titrate the virus directly. The steps of virus titration are as follows: the virus is diluted 5 times in...
Embodiment 3
[0083] Example 3. CHFI polypeptide has significant activity against HIV drug-resistant strains
[0084] Amino acid mutations in the NHR region of HIV fusion protein gp41 can lead to virus resistance to fusion inhibitors. To further evaluate the anti-HIV activity of CHFI, we used a set of HIV gp41 natural mutant strains and induced mutant strains in Table 4 to test the inhibitory effect of CHFI on viral infection. Natural mutant strains include L33S, L33M, L33V, S35F, Q39R, L54M, Q56K, Q56R, L54M / Q56K, L54M / Q56R and L34M / L54M / Q56R, the preparation method of which is shown in the published literature of people such as Chinnadurai (Raghavan Chinnadurai, Jan Mu¨nch and Frank Kirchhoff. Effect of naturally-occurring gp41 HR1 variations on susceptibility of HIV-1 to fusion inhibitors. AIDS 2005, 19: 1401-1405); induced mutants include I37Q / V38M, I37Q / V38Q, I37S / V38N and I37V / V38T, its preparation method is shown in the literature published by Chinnadurai et al. Ex Vivo Human Lym...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com