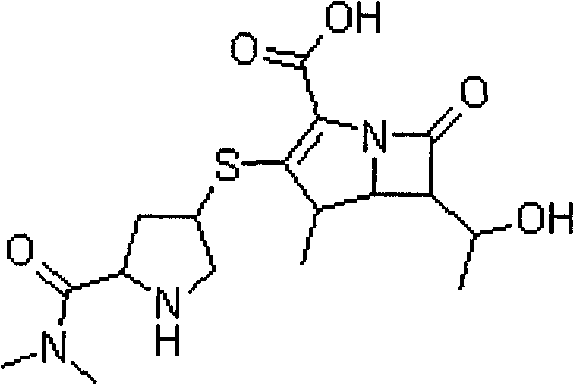
Stable meropenem injection and preparation method thereof
A technology for meropenem and injection, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, and pharmaceutical formulas, etc. The effect of low leakage rate, solution instability and high encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Meropenem liposome freeze-dried powder for injection
[0048] 1. Prescription:
[0049] components
weight
weight percentage (%)
Meropenem
8g
0.27
60g
2.01
Phosphatidylglycerol Distearate
30g
1.00
60g
2.01
Vitamin E
150mg
0.01
sodium deoxycholate
300g
10.04
glucose
30g
1.00
Water for Injection
2500g
83.66%
[0051] 2. Preparation process
[0052] 1) Preparation of blank liposomes: Weigh the amount of egg yolk lecithin, phosphatidylglycerol distearate, cholesterol, vitamin E and sodium deoxycholate, add an appropriate amount of chloroform-dehydrated ethanol mixed solvent (volume ratio 7: 1) dissolving, decompression evaporates solvent, makes the lipid film of uniform dryness, then adds the citric acid-sodium citrate buffer solution of 0....
Embodiment 2
[0064] Example 2: Meropenem liposome freeze-dried powder for injection
[0065] 1. Prescription:
[0066] components
weight
weight percentage (%)
Meropenem
13g
0.39%
100g
3.00%
Phosphatidylglycerol Distearate
55g
1.65%
cholesterol
103g
3.09%
Vitamin E
250mg
0.01%
515g
15.44%
glucose
50g
1.50%
Water for Injection
2500g
74.93%
[0067] 2. Preparation process
[0068] 1) Preparation of blank liposomes: Weigh the amount of soybean lecithin, phosphatidylglycerol distearate, cholesterol, vitamin E and sodium deoxycholate, add an appropriate amount of chloroform-dehydrated alcohol mixed solvent (volume ratio 5:1) was dissolved, and the solvent was evaporated under reduced pressure to form a uniform dry lipid film, and then 0.1mol / L citric acid-sodium citrate buffer solution w...
Embodiment 3
[0080] Example 3: Meropenem liposome freeze-dried powder for injection
[0081] 1. Prescription:
[0082] components
weight
weight percentage (%)
Meropenem
13g
0.40%
96g
2.92%
Dimyristate Phosphatidylglycerol
50g
1.52%
cholesterol
97.5g
2.96%
Vitamin E
500mg
0.02%
487.5g
14.81%
glucose
46.25g
1.41%
Water for Injection
2500g
75.97%
[0083] 2. Preparation process
[0084] 1) Preparation of blank liposomes: Weigh the amount of egg yolk lecithin, dimyristate phosphatidylglycerol, cholesterol, vitamin E and sodium deoxycholate, add an appropriate amount of chloroform-absolute ethanol mixed solvent (volume ratio 5.5:1) was dissolved, and the solvent was evaporated under reduced pressure to form a uniform dry lipid film, and then 0.1mol / L citric acid-sodium citrate buff...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com