Micelle loaded with Tacrolimus or pharmaceutical salts thereof, lyophilized preparation as well as preparation methods and applications
A technology of tacrolimus and freeze-dried preparations, which is applied in the field of pharmaceutical dosage forms and nano-medicine preparations, can solve the problems of neurotoxicity, insensitivity, inability to move, mutism, seizures, etc., and achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] (1) 85 mg of methoxypolyethylene glycol poly(ε-caprolactone) polymer (MPEG 2000 -PCL 2000 ) and 15 mg of tacrolimus were dissolved in 2 ml of absolute ethanol, heated and stirred until completely dissolved to obtain a clear solution A;
[0085] (2) Under heating conditions, the clear solution A is rotary evaporated to remove the organic solvent to make it a transparent gel B;
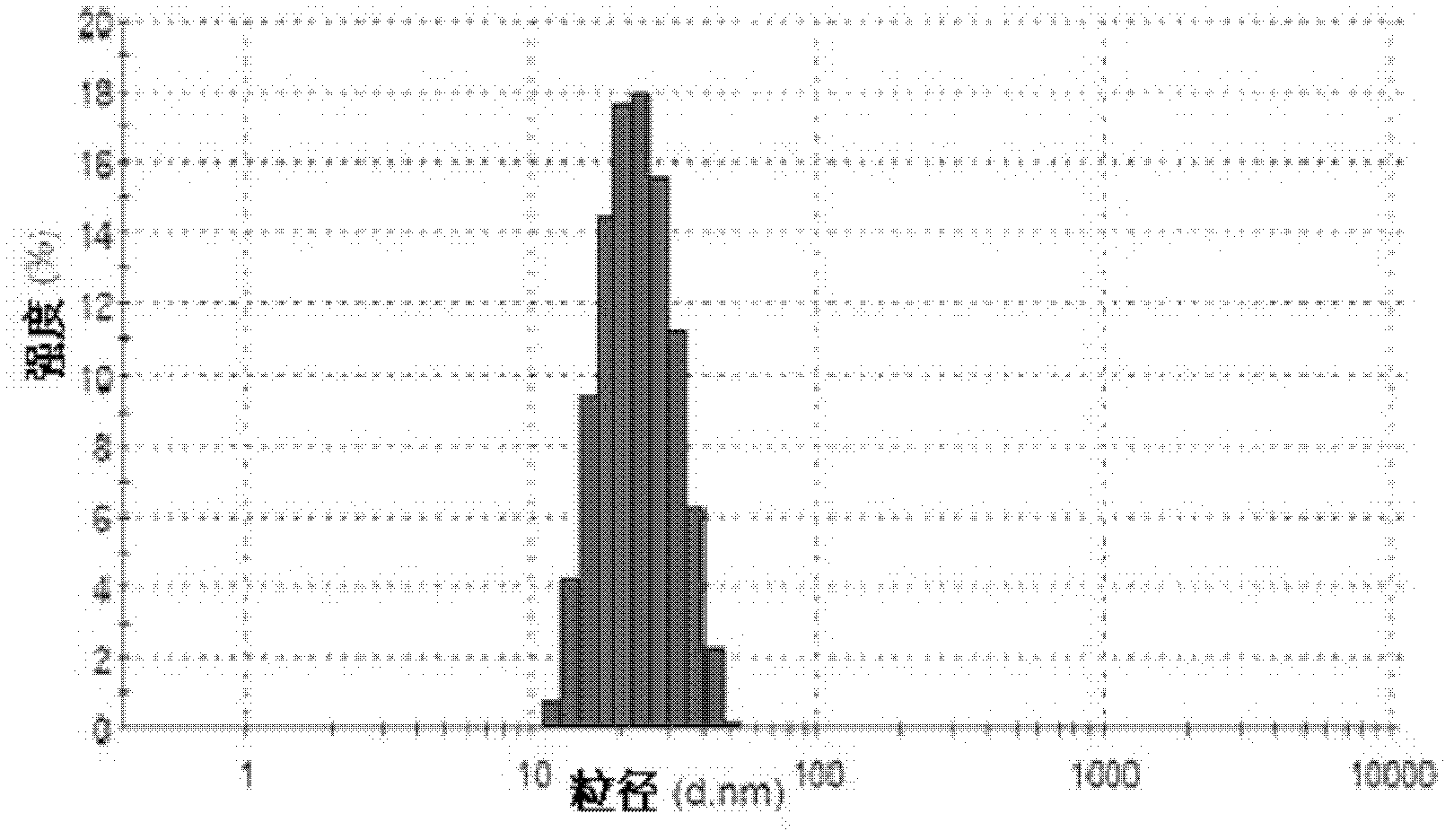
[0086] (3) Add 5ml of preheated water for injection into gel B, make it clear and transparent under the condition of heating and stirring, and pass through a 0.22 μm filter membrane to obtain micelles C with a particle size of 10nm-100nm;
[0087](4) Freeze-dry the micelles C to obtain the final stable nano-medicine freeze-dried powder D; before the clinical use of the freeze-dried powder, adding water for injection will automatically form a sustained-release nano-sized glue under the condition of heating. bundle.
[0088] The obtained micelles C, lyophilized powder D and final reconstituted m...
Embodiment 2
[0090] (1) 92 mg of methoxypolyethylene glycol poly(ε-caprolactone) polymer (MPEG 2000 -PCL 2000 ) and 8 mg of tacrolimus were dissolved in 2 ml of absolute ethanol, heated and stirred until completely dissolved to obtain a clear solution A;
[0091] (2) Under heating conditions, the clear solution A is rotary evaporated to remove the organic solvent to make it a transparent gel B;
[0092] (3) Add 5ml of preheated water for injection into gel B, make it clear and transparent under the condition of heating and stirring, and pass through a 0.22 μm filter membrane to obtain micelles C with a particle size of 10nm-100nm;
[0093] (4) Freeze-dry the micelles C to obtain the final stable nano drug freeze-dried powder D.
[0094] Before the clinical use of the lyophilized powder, adding water for injection will automatically form sustained-release nano-sized micelles under the condition of heating.
[0095] The obtained micelles C, lyophilized powder D and the final reconstituted...
Embodiment 3
[0097] (1) 92mg poly(ε-caprolactone)-polyethylene glycol-poly(ε-caprolactone) triblock polymer (PCL 850 -PEG 2000 -PCL 850 ) and 8 mg of tacrolimus were dissolved in 2 ml of absolute ethanol, heated and stirred until completely dissolved to obtain a clear solution A;
[0098] (2) Under heating conditions, the clear solution A is rotary evaporated to remove the organic solvent to make it a transparent gel B;
[0099] (3) Add 5ml of preheated water for injection into gel B, make it clear and transparent under the condition of heating and stirring, and pass through a 0.22 μm filter membrane to obtain micelles C with a particle size of 10nm-100nm;
[0100] (4) Freeze-dry the micelles C to obtain the final stable nano-medicine freeze-dried powder D; before the clinical use of the freeze-dried powder, adding water for injection will automatically form a sustained-release nano-sized glue under the condition of heating. bundle.
[0101] The drug loading of the obtained micelles wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com