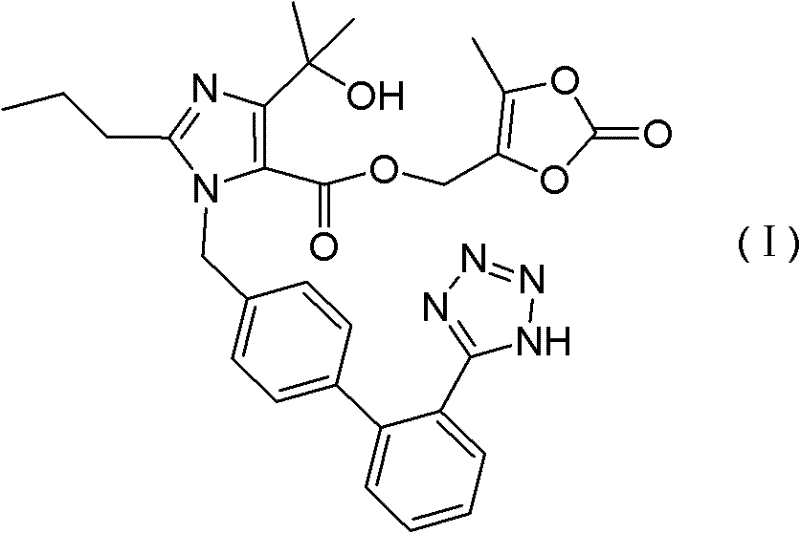
Preparation method for olmensartan medoxomil with low-level impurity
A technology of olmesartan medoxomil and esterification reaction, which is applied in the production of organic chemistry and bulk chemicals, can solve the problems of high price, high toxicity, unfavorable industrial production, etc., and achieve the goal of avoiding the use of highly toxic solvents and mild conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
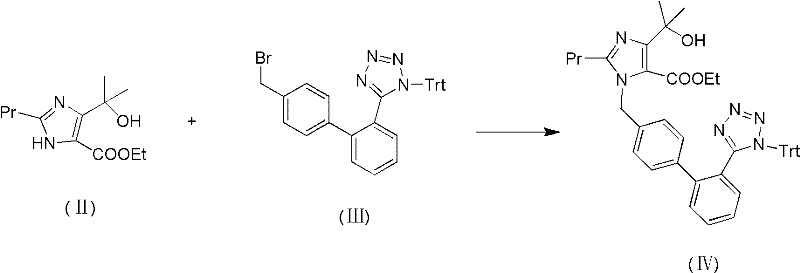
[0024] 1-[[[2′-(trityl)-2H-tetrazol-5-yl]biphenyl-4-yl]methyl]-2-propyl-4-(1-hydroxyl-1- Preparation and Purification of Ethyl Methyl)imidazole-5-carboxylate (IV)
[0025] 7.0g calcium oxide, 15.0g 4-(1-hydroxyl-1-methylethyl)-2-propylimidazole-5-carboxylate ethyl ester (II) and 38.30g 4-[2-(trityl tetrazole -5-yl)phenyl]benzyl bromide (III), suspended in 90ml of N,N-dimethylacetamide, heating the reaction mixture to 60°C to 65°C, insulated and stirring the reaction until the end of the reaction (3~ 5 hours). The reaction solution was filtered hot, and the filtrate was cooled to 20°C and then poured into 400ml of ice water. A large amount of solids were precipitated, filtered and dried to obtain 49.60g of crude product (IV).
[0026] Add the crude product of (IV) to 300ml of acetone, heat to reflux, and after dissolving, heat filter, and the filtrate is gradually cooled to 20°C, kept at this temperature for 3 hours, then continued to cool to 0°C to 5°C, and kept for 2 hours...
Embodiment 2
[0029] 1-[[[2′-(trityl)-2H-tetrazol-5-yl]biphenyl-4-yl]methyl]-2-propyl-4-(1-hydroxyl-1- Preparation and Purification of Ethyl Methyl)imidazole-5-carboxylate (IV)
[0030] 11.2g alumina, 17.6g ethyl 4-(1-hydroxy-1-methylethyl)-2-propylimidazole-5-carboxylate (II) and 45.0g 4-[2-(trityl tetrazole -5-yl)phenyl]benzyl bromide (III), suspended in 100ml N,N-dimethylacetamide, heating the reaction mixture to 60°C to 65°C, keeping the temperature and stirring the reaction until the end of the reaction (3~ 5 hours). The reaction solution was filtered hot, and the filtrate was cooled to 20°C and then poured into 450ml of ice water. A large amount of solids were precipitated, filtered and dried to obtain 52.60g of crude product (IV).
[0031] Add the crude product of (IV) to 300ml of acetone, heat to reflux, and after dissolving, heat filter, and the filtrate is gradually cooled to 20°C, kept at this temperature for 3 hours, then continued to cool to 0°C to 5°C, and kept for 2 hours,...
Embodiment 3
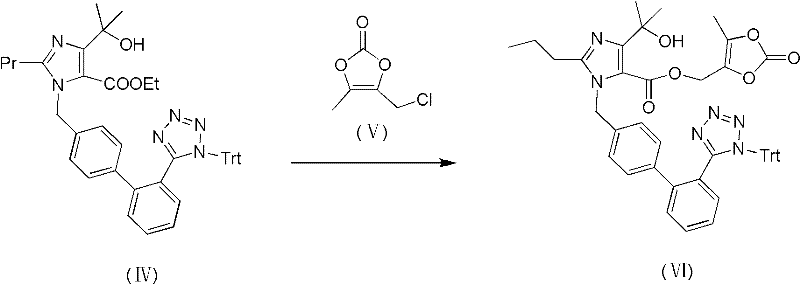
[0038] 1-[[[2′-(trityl)-2H-tetrazol-5-yl]biphenyl-4-yl]methyl]-2-propyl-4-(1-hydroxyl-1- Preparation and Purification of Methylethyl)imidazole-5-carboxylic acid (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl ester (VI)
[0039]36.0 g of high-purity compound (IV) and 3.0 g of sodium hydroxide powder were suspended in 270 ml of dimethylacetamide, stirred at a temperature of 30°C to 35°C for 3 hours, and compound (IV) was completely reacted. The reaction mixture was cooled to 0°C, 6.90 g of potassium carbonate was added, and under stirring, 13.91 g of 4-chloromethyl-5-methyl-2-oxo-1,3 - Dioxole (V), after the addition is completed, the reaction mixture is slowly heated to 60° C. to 65° C., and the reaction is kept for 3 to 5 hours. After the reaction, the reaction mixture was cooled to 5°C-10°C, poured into 800ml of ice water, a large amount of solid precipitated, filtered, and dried to obtain 43.10g of crude product (VI).
[0040] Add the crude product of (VI) to 150ml of acetone, hea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com