Preparation method of zinc aluminum silicate glass codoped with zinc oxide nanocrystals and rare-earth ions
A technology of zinc oxide nanocrystals and silicate glass, which is applied in the field of preparation of zinc oxide nanocrystals and rare earth ions co-doped zinc aluminum silicate glass, to achieve the effects of optimized optoelectronic properties, uniform size, and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 1) Put zinc oxide powder, silicon dioxide, aluminum oxide, potassium oxide and europium oxide into a mortar, mix well and pour into a crucible, zinc oxide powder, silicon dioxide, aluminum oxide, potassium oxide and europium oxide The mass ratio is 35:35.8:15.2:14:0.2;
[0017] 2) Put the crucible containing the batch material into a high-temperature furnace, raise the temperature to 1560°C at a heating rate of 10°C / min, and keep it for 2 hours to melt;
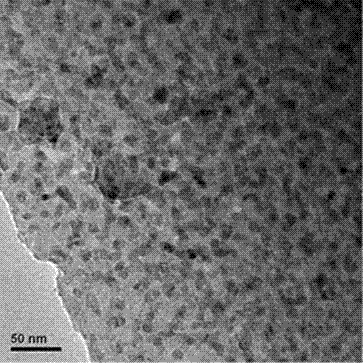
[0018] 3) The molten solution in step 2) was poured on a copper plate at 300°C to cool to room temperature, and then heat-treated at 650°C for 2 h to obtain a zinc-aluminosilicate glass co-doped with zinc oxide nanocrystals and rare earth ions.
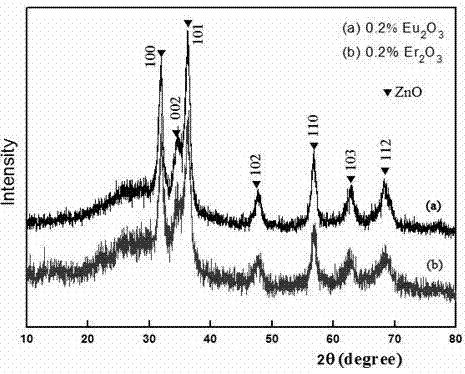
[0019] The X-ray diffraction pattern of the product is shown in the curve (a) of Figure 1. It can be seen from the figure that the diffraction peaks of the sample are consistent with the standard pattern of zinc oxide with wurtzite structure, indicating that the obtained produ...
Embodiment 2
[0021] 1) Put zinc oxide powder, silicon dioxide, aluminum oxide, potassium oxide and erbium oxide into a mortar, mix well and pour into a crucible, zinc oxide powder, silicon dioxide, aluminum oxide, potassium oxide and erbium oxide The mass ratio is 35:35.8:15.2:14:0.2;
[0022] 2) Put the crucible containing the batch material into a high-temperature furnace, raise the temperature to 1560 °C at a heating rate of 7 °C / min, and keep it for 2 h to melt;
[0023] 3) The molten solution in step 2) was poured on a graphite plate at 500 °C to cool to room temperature, and then heat-treated at 700 °C for 2 h to obtain a zinc-aluminosilicate glass co-doped with zinc oxide nanocrystals and rare earth ions.
[0024] The X-ray diffraction pattern of the product is shown in Figure 1 curve (b), and its diffraction peaks are consistent with the standard pattern of wurtzite structure zinc oxide, indicating that the obtained product is a wurtzite structure zinc oxide nanocrystal. Calculate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Grain diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com