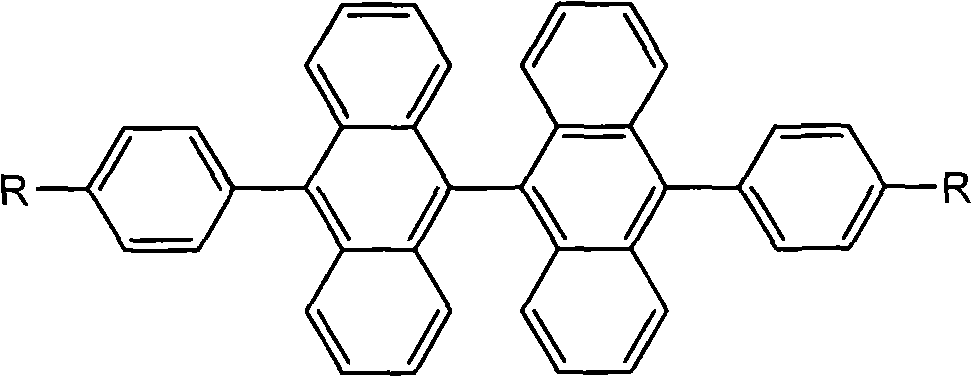
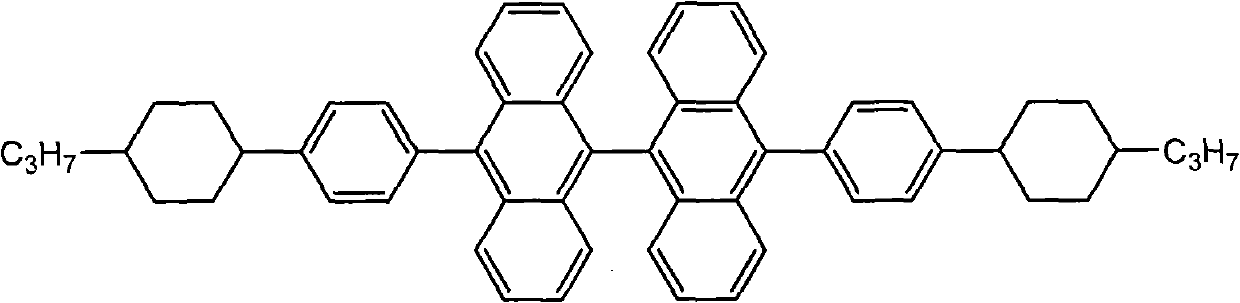
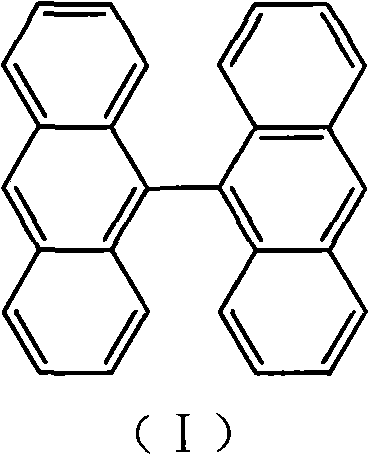
9,9<,>-bisanthracene derivative and preparation method thereof
A derivative, bianthracene technology, applied in the field of blue organic electroluminescent materials, can solve the problems of less variety and the need to improve the fluorescence quantum efficiency of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1,10, the synthesis of 10'-diphenyl-9,9'-bianthracene
[0023] The present invention is implemented according to the following synthetic route
[0024]
[0025] 1) Synthesis of phenylboronic acid
[0026] In a 250mL round-bottomed bottle, add magnesium powder (0.72g, 0.03mol), bromobenzene (1.57g, 0.01mol), tributyl borate (4g, 0.02mol), and 20mL of tetrahydrofuran dried through molecular sieves. Condenser. Ultrasonic radiation was carried out after heating to 40° C. in an ultrasonic cleaner, and the reaction was completed after 1 hour. Add 2mol·L -1 5mL of hydrochloric acid, and after stirring for a while, the organic layer was separated. The aqueous layer was extracted with ethyl acetate (10 mL×2), several layers were combined and washed with water until neutral, dried and the solvent was removed to obtain a crude product. Recrystallization was carried out with ethanol: water = 2: 1, and 0.87 g of solid powder was obtained after drying under reduced...
Embodiment 2
[0036] Embodiment 2,10, the synthesis of 10'-bis(4-ethyl)phenyl-9,9-bianthracene
[0037] The present invention is implemented according to the following synthetic route
[0038]
[0039] 1) Synthesis of p-ethylphenylboronic acid
[0040] In a 250mL round bottom bottle, add magnesium powder (0.72g, 0.03mol), 4-ethylbromobenzene (1.85g, 0.01mol), tributyl borate (4g, 0.02mol), and 20mL of tetrahydrofuran dried over molecular sieves , install the reflux condenser. Ultrasonic radiation was carried out after heating to 40° C. in an ultrasonic cleaner, and the reaction was completed after 1 hour. Add 2mol·L -1 5mL of hydrochloric acid, and after stirring for a while, the organic layer was separated. The aqueous layer was extracted with ethyl acetate (10 mL×2), several layers were combined and washed with water until neutral, dried and the solvent was removed to obtain a crude product. Recrystallization was carried out with ethanol: water = 2: 1, and 1.12 g of solid powder w...
Embodiment 3
[0049] Embodiment 3,10, the synthesis of 10'-two (4-propyl) phenyl-9,9'-bianthracene
[0050] The present invention is implemented according to the following synthetic route
[0051]
[0052] 1) Synthesis of p-propylphenylboronic acid
[0053] In a 250mL round bottom bottle, add magnesium powder (0.72g, 0.03mol), 4-propyl bromobenzene (2g, 0.01mol), tributyl borate (4g, 0.02mol), 20mL of tetrahydrofuran dried through molecular sieves, Install the reflux condenser. Ultrasonic radiation was carried out after heating to 40° C. in an ultrasonic cleaner, and the reaction was completed after 1 hour. Add 2mol·L -1 5mL of hydrochloric acid, and after stirring for a while, the organic layer was separated. The aqueous layer was extracted with ethyl acetate (10 mL×2), several layers were combined and washed with water until neutral, dried and the solvent was removed to obtain a crude product. Recrystallization was carried out with ethanol: water = 2: 1, and 1.11 g of solid powder...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com